Beauty Ingredient Blacklist: Harmful Chemicals Still Legal in Your Favorite Products

Table of Contents
- Introduction: The Morning My Lipstick Made Me Question Everything
- 1. The Shocking Truth About Beauty Product Regulation
- 2. Parabens: The Hormone Disruptors Hiding in Plain Sight
- 3. Phthalates: Reproductive Harm in Your Nail Polish
- 4. Formaldehyde: Cancer-Causing Preservatives in Your Shampoo
- 5. PFAS Forever Chemicals: The Toxins That Never Leave
- 6. Heavy Metals: Lead and Arsenic in Your Lipstick
- 7. Synthetic Fragrances: Toxic Cocktails Behind One Word
- 8. Chemical Sunscreens: Protection or Poison
- 9. How to Read Ingredient Labels Like an Expert
- 10. Building Your Safe Beauty Routine
- Conclusion: Your Health Deserves Better Than Toxic Beauty
- Frequently Asked Questions
Introduction: The Morning My Lipstick Made Me Question Everything
The tube of lipstick sat innocently on my bathroom counter where it had resided for the past three months, a premium shade of deep rose that I’d splurged forty-two dollars on after the beauty influencer I followed raved about its creamy texture and all-day staying power. I applied it every morning as part of my routine, swiping the color across my lips without a second thought beyond whether it complemented my outfit and if I needed to blot excess product onto tissue. That Tuesday morning started like countless others—alarm at six-thirty, shower, skincare, makeup—until I paused mid-application of that expensive lipstick and actually read the ingredient list for the first time since purchasing it months earlier.
The tiny print covering the bottom of the tube listed dozens of chemical names I couldn’t pronounce, substances I’d never heard of despite using this product daily for weeks, compounds that might as well have been written in a foreign language for all the meaning they conveyed. But one ingredient jumped out with alarming familiarity: lead acetate. I stood frozen with the lipstick hovering inches from my mouth, my mind racing through fragmented memories of childhood warnings about lead paint and news reports about lead contamination in water supplies causing developmental delays and neurological damage. Lead. In my lipstick. The premium luxury lipstick I’d carefully researched through beauty blogs and review videos before spending what felt like an irresponsible amount of money for a single cosmetic item.
My hands trembled slightly as I grabbed my phone and started searching, my initial assumption being that I must have misread or misunderstood the ingredient list because surely cosmetic companies wouldn’t put actual lead in products people apply to their mouths multiple times daily. The search results that flooded my screen during the next fifteen minutes shattered assumptions I’d held unconsciously for decades about beauty product safety, consumer protection, and the basic expectation that products sold legally in stores must be reasonably safe for their intended use. Not only did my expensive lipstick contain lead—apparently common in color cosmetics despite the metal’s well-documented toxicity—but the presence of this neurotoxic heavy metal in lip products was perfectly legal, requiring no warning labels, no disclosure beyond the technical ingredient list most people never read, and no regulatory action despite decades of scientific research documenting lead’s harmful effects even at low exposure levels.
The rabbit hole deepened disturbingly as my frantic morning research revealed that lead represented just one of dozens of potentially harmful chemicals routinely included in cosmetics despite evidence linking them to cancer, hormone disruption, reproductive harm, developmental delays, organ damage, and numerous other serious health conditions. Parabens acting as endocrine disruptors that mimic estrogen in the body. Phthalates associated with reproductive system abnormalities and fertility problems. Formaldehyde-releasing preservatives classified as human carcinogens. PFAS forever chemicals that accumulate in organs and never break down. Synthetic fragrances serving as catch-all terms concealing dozens of undisclosed chemical compounds. The list continued with disturbing length and severity, each ingredient revelation more shocking than the last as I scrolled through scientific studies, consumer safety reports, and investigative journalism exposing the inadequate regulation allowing these substances in products millions of people use daily.
The most disturbing discovery involved learning that cosmetic safety regulations in the United States—where I live and purchase products—remain shockingly inadequate compared to other developed nations, with the FDA having banned only eleven ingredients for use in cosmetics versus over sixteen hundred substances prohibited in the European Union. This regulatory disparity means that products perfectly legal to sell in American stores cannot be marketed in European countries due to toxic ingredients those nations recognized as unacceptably dangerous based on scientific evidence. The lipstick sitting on my counter, along with most of the other beauty products crowding my bathroom shelves, likely couldn’t be sold in dozens of countries with stronger consumer protections, yet American regulations allowed manufacturers to market these formulations with minimal safety testing and no requirement to prove ingredients were safe before putting products on shelves where consumers like me purchased them assuming someone somewhere had verified they wouldn’t cause harm.
The betrayal I felt that morning extended beyond simple disappointment with a single product to fundamental questions about trust, safety, and the systems supposedly protecting consumers from harmful products. How could products I used daily on my skin, lips, and hair—products marketed specifically for beautification and self-care—contain chemicals associated with cancer, hormone disruption, and organ damage? Why would manufacturers choose to include these ingredients when safer alternatives existed? How had I remained ignorant about these dangers despite being a relatively informed consumer who read reviews, researched brands, and made conscious purchasing decisions? The answers I discovered over the following days and weeks revealed a troubling intersection of inadequate regulation, industry lobbying preventing reform, misleading marketing creating false impressions of safety, and consumer ignorance perpetuated by the complexity of cosmetic chemistry and overwhelming volume of products each containing dozens of ingredients with technical names impossible for laypeople to evaluate.
My bathroom counter transformation from a simple cosmetics collection into what felt like a toxic waste site happened gradually as I researched each product, reading ingredient lists I’d previously ignored and discovering that nearly every item contained at least one and usually multiple substances flagged as concerning by safety organizations, banned in other countries, or linked to health risks by scientific research. The premium moisturizer contained parabens and phthalates. The volumizing mascara included formaldehyde-releasing preservatives. The silky foundation listed PFAS chemicals that persist indefinitely in the environment and human body. The lovely botanical shampoo featured synthetic fragrances concealing undisclosed compounds potentially as harmful as the disclosed ingredients. Even the clean beauty brands I’d started purchasing after becoming concerned about conventional cosmetics proved disappointing when deeper investigation revealed misleading natural labels, greenwashing marketing, and the presence of questionable ingredients despite advertising suggesting otherwise.
The statistics I uncovered during my research painted a disturbing picture of widespread chemical exposure through daily beauty routines most people never question. The average woman uses twelve personal care products daily containing one hundred sixty-eight unique chemical ingredients according to studies by environmental health organizations, while men use an average of six products containing eighty-five chemicals. Many of these substances lack adequate safety data, have never been tested for long-term health effects, and remain completely unregulated despite being applied to skin—the body’s largest organ with significant absorption capacity—or used near mucous membranes allowing chemicals direct access to bloodstream without digestive system filtering. The bioaccumulation of these chemicals in human tissue has been documented through biomonitoring studies finding cosmetic ingredients in blood, urine, breast milk, and organs of virtually every person tested, creating body burdens of synthetic chemicals that didn’t exist in human history until recent decades of expanding cosmetic use.
The health implications of this widespread chemical exposure remain inadequately studied but increasingly concerning as research links cosmetic ingredients to rising rates of hormonally-driven cancers, declining fertility rates in developed nations, earlier onset of puberty in girls, and increasing prevalence of conditions associated with endocrine disruption. While establishing direct causal relationships between specific cosmetic ingredients and health outcomes proves methodologically challenging due to the complexity of multiple chemical exposures from various sources, the precautionary principle suggests that evidence of potential harm should warrant protective action rather than waiting for conclusive proof of damage after widespread exposure has occurred. Yet cosmetic regulations in most countries—particularly the United States—operate on the opposite assumption, allowing ingredients to be used freely until definitive evidence of harm accumulates sufficiently to force regulatory action, by which point millions of people have already been exposed to substances that are eventually recognized as dangerous.
This comprehensive guide emerged from my journey from naive consumer trusting that legal products must be safe to informed advocate demanding better from the beauty industry and regulatory systems meant to protect public health. We’ll explore the specific ingredients research has identified as most concerning, examine why these substances remain legal despite evidence of harm, investigate the regulatory failures enabling toxic chemicals in cosmetics, and provide practical guidance for identifying and avoiding these ingredients while building safer beauty routines. The goal involves empowering informed decisions and driving consumer demand for genuinely safe products that ultimately forces industry change even when regulation fails to protect us adequately.
Different readers will approach this information with varying levels of concern and willingness to change established beauty routines. Some will feel compelled to immediately discard all conventional cosmetics and rebuild their entire regimen with carefully vetted safe alternatives. Others will take measured approaches focusing on replacing high-risk products while accepting that perfect safety remains impossible in our chemical-saturated world. Still others might read this information and consciously decide that they accept the risks associated with conventional products in exchange for the performance, aesthetics, or convenience those formulations provide. All these responses represent valid personal choices, but they should be informed choices based on understanding the actual risks rather than unconscious acceptance of whatever manufacturers decide to put in products based primarily on cost considerations and performance characteristics with minimal attention to long-term health effects.
The investment of time required to become informed about cosmetic ingredient safety and rebuild your beauty routine with safer alternatives varies dramatically depending on how extensively you use beauty products and how thoroughly you choose to investigate options. Basic awareness of the highest-risk ingredients to avoid can be achieved in a single afternoon of focused research. Thoroughly vetting your entire existing collection and identifying specific concerning ingredients in each product might take several days spread across weeks. Researching safer alternatives, evaluating brands making genuine safety commitments versus those engaged in greenwashing, and rebuilding your complete beauty routine could require months of ongoing effort. But the time investment pays returns through reduced exposure to chemicals that may compromise your health, the satisfaction of making informed conscious choices rather than passive consumption, and the contribution your purchasing decisions make toward shifting market demand toward safer formulations.
This guide is structured to provide both practical product selection guidance and deeper understanding of cosmetic ingredient science and regulatory contexts that extend beyond simple lists of good and bad chemicals. If you need immediate answers about which specific ingredients to avoid, jump directly to sections covering the chemical categories of greatest concern. If you want foundational understanding of why these substances remain legal despite known risks and what distinguishes genuine safety concerns from exaggerated fears, read sequentially to build comprehensive knowledge enabling independent evaluation of products and claims. Throughout, we’ll maintain honesty about uncertainties in the science, acknowledge legitimate debates about risk levels, and distinguish between chemicals with strong evidence of harm versus those with more speculative concerns.
The journey from unconscious consumer to informed advocate involves more than simply memorizing ingredient names to avoid—it requires understanding the complex interplay between cosmetic chemistry, regulatory policy, industry practices, and personal health priorities that together determine what products reach market and which formulations you ultimately choose to use. Some people find empowerment through knowledge and active ingredient avoidance. Others feel overwhelmed by the complexity and paralyzed by the impossibility of perfect safety in a world where chemicals pervade virtually everything. Neither response is wrong, but understanding both the real risks and the practical limitations of what individuals can control helps identify approaches that genuinely improve safety rather than simply creating anxiety without meaningful harm reduction.
Your skin, your health, and your body deserve better than serving as an uncontrolled experiment testing the long-term effects of chemical combinations that have never been adequately studied for safety. The beauty industry deserves accountability for the formulations they choose to market based on profit considerations rather than health protection. Regulatory systems deserve reform to close the massive gaps allowing toxic chemicals in products people use daily. But until those systemic changes occur—and they will only occur through sustained pressure from informed consumers demanding better—you must protect yourself through knowledge, careful product selection, and strategic choices about which risks you accept versus which exposures you can effectively minimize.
Let’s explore the specific ingredients that research and regulatory bodies internationally have identified as most concerning, understand why they remain legal in many products despite these concerns, and develop practical frameworks for navigating the complex landscape of cosmetic ingredient safety in a world where your bathroom routine shouldn’t require a chemistry degree to make reasonably safe choices.
1. The Shocking Truth About Beauty Product Regulation
The foundation of cosmetic ingredient problems lies in the shocking inadequacy of regulatory oversight governing what manufacturers can legally include in products marketed for daily use on human skin and bodies. Most consumers operate under the reasonable but incorrect assumption that products sold legally in stores must have undergone rigorous safety testing, that potentially harmful ingredients would be prohibited by government agencies tasked with consumer protection, and that cosmetic companies bear legal responsibility for ensuring their formulations don’t cause harm to people using products as directed. These assumptions prove dangerously inaccurate under the actual regulatory framework governing cosmetics in most countries—particularly the United States where regulations remain frozen in the distant past despite decades of scientific advancement in understanding chemical toxicity and long-term health effects.
The FDA's Minimal Cosmetic Authority
The Food and Drug Administration in the United States operates under the Federal Food, Drug, and Cosmetic Act passed in 1938—a law written almost ninety years ago when understanding of chemical toxicity and long-term health effects was primitive compared to current scientific knowledge. This outdated legal framework provides the FDA with remarkably limited authority over cosmetics compared to the extensive powers the agency wields over drugs and medical devices. Cosmetic manufacturers are not required to register their facilities or products with the FDA before bringing them to market, submit safety data demonstrating their formulations are safe for intended uses, disclose ingredient lists to regulatory authorities, or report adverse event complaints from consumers experiencing health problems potentially linked to product use.
The FDA has banned or restricted only eleven ingredients or ingredient classes for use in cosmetics—a list including bithionol, chlorofluorocarbon propellants, chloroform, halogenated salicylanilides, hexachlorophene, mercury compounds, methylene chloride, prohibited cattle materials, and vinyl chloride. This list pales embarrassingly compared to the European Union’s prohibition of over sixteen hundred substances, Canada’s ban on roughly six hundred ingredients, and increasingly strict regulations implemented by many Asian nations following decades of scientific research identifying chemicals with evidence of serious health risks. Products perfectly legal to manufacture and sell in American stores often cannot be marketed in dozens of other developed nations due to toxic ingredients those countries recognize as unacceptably dangerous based on the same scientific literature available to American regulators.
The burden of proof under current American regulations falls perversely on the FDA to definitively demonstrate that an ingredient causes harm before the agency can take action restricting its use, rather than requiring manufacturers to prove safety before including substances in products people will apply to their bodies. This means chemicals can be widely used in cosmetics for decades while evidence of harm slowly accumulates through scientific research, during which time millions of people get exposed to substances that will eventually be recognized as dangerous but only after the damage is done. Even when concerning evidence does emerge, the legal and political process of translating that evidence into actual regulatory restrictions proves lengthy and contentious as industry groups challenge any restrictions using their substantial lobbying resources and legal teams to protect profitable ingredients regardless of health implications.
International Regulatory Comparisons
The stark contrast between American cosmetic regulations and international standards reveals just how inadequate consumer protections are in the United States despite the nation’s wealth and scientific capabilities. The European Union’s cosmetic regulations require pre-market safety assessments conducted by qualified safety assessors, prohibit over sixteen hundred specific ingredients identified as carcinogens, mutagens, reproductive toxicants, or otherwise harmful, mandate reporting of serious adverse effects, and require detailed ingredient labeling including nanomaterials and allergen disclosure. These comprehensive protections reflect the precautionary principle where potential risks warrant preventive action rather than waiting for conclusive evidence of harm after widespread exposure has occurred.
Canada’s cosmetic regulations similarly prohibit roughly six hundred substances based on health and safety concerns while requiring cosmetic manufacturers to report product information including ingredient lists and adverse reactions to Health Canada. Japan’s cosmetic laws establish positive lists of permitted ingredients rather than prohibited lists, meaning substances must be specifically approved for cosmetic use rather than allowed freely until evidence forces restrictions. South Korea’s cosmetics act requires safety assessments before products launch, maintains prohibited and restricted ingredient lists, and implements robust post-market surveillance including mandatory adverse event reporting. Australia’s cosmetic regulations align largely with EU standards, prohibiting similar ingredients and requiring compliance with comprehensive safety assessment protocols.
The cumulative effect of these international regulatory differences creates a perverse situation where American consumers—citizens of one of the wealthiest nations with extensive public health infrastructure—actually receive less protection from potentially harmful cosmetic ingredients than people living in dozens of other countries with varying income levels but stronger commitment to precautionary consumer protection. This regulatory failure doesn’t reflect scientific uncertainty about these ingredients since international regulators restricting them cite the same scientific literature available to American agencies, but rather reflects political choices about how to balance industry interests against public health concerns. American regulations overwhelmingly prioritize industry freedom and minimal government interference even when that approach leaves consumers exposed to chemicals other nations consider too dangerous for cosmetic use.
The practical implication involves recognizing that ingredient legality in American cosmetics provides virtually no assurance of safety since the regulatory bar remains set so extraordinarily low that almost anything short of acute poisoning passes without restriction. An ingredient being legal doesn’t mean it has been tested and determined safe—it simply means that the FDA hasn’t accumulated sufficient political will to restrict it despite any scientific evidence of concern that might exist. This places responsibility squarely on consumers to educate themselves and make informed choices rather than relying on regulatory oversight that fundamentally fails to protect them adequately.
Shop on AliExpress via link: wholesale-clean-beauty-products
2. Parabens: The Hormone Disruptors Hiding in Plain Sight
Parabens represent perhaps the most widely discussed and debated cosmetic preservatives, appearing in an estimated seventy-five to ninety percent of conventional beauty products despite growing evidence linking these synthetic chemicals to hormone disruption, reproductive harm, and potential contributions to hormonally-driven cancers. These preservatives—identified on ingredient lists with names like methylparaben, propylparaben, butylparaben, and ethylparaben—prevent bacterial and fungal growth in cosmetics containing water, extending product shelf life and preventing contamination that could cause infections or product degradation. Their antimicrobial effectiveness, low cost, and decades of established use made parabens the default preservative choice for cosmetic manufacturers, creating ubiquitous exposure through daily beauty routines most consumers never questioned until scientific research began revealing concerning endocrine disruption properties.
How Parabens Disrupt Hormones
The mechanism by which parabens cause concern involves their ability to mimic estrogen in the human body—binding to estrogen receptors on cells and triggering estrogenic activity despite being synthetic chemicals rather than actual hormones. This endocrine disruption potentially affects numerous body systems since estrogen plays critical roles far beyond reproduction, influencing bone density, cardiovascular health, brain function, and metabolism throughout life. Even low-level exposure to compounds mimicking estrogen can alter the delicate balance of hormone signaling, particularly during developmentally sensitive periods like fetal development, infancy, childhood, and puberty when hormone levels normally change in precise patterns essential for proper growth and maturation.
Research measuring parabens’ estrogenic potency finds these preservatives approximately ten thousand to one million times weaker than natural estrogen, leading industry advocates to argue that exposure levels from cosmetics are too low to cause meaningful effects. However, this comparison proves misleading because unlike natural estrogen which the body carefully regulates and metabolizes, synthetic parabens accumulate in tissues where they exert continuous low-level estrogenic activity without normal regulatory mechanisms controlling their concentration or duration. The combination of multiple parabens from different products creates additive exposure that individual product testing doesn’t capture, while co-exposure to other endocrine disrupting chemicals from various sources potentially creates synergistic effects where total impact exceeds the sum of individual chemical exposures.
Human biomonitoring studies consistently detect parabens in urine samples from virtually everyone tested in developed nations, confirming widespread exposure primarily through cosmetic use and demonstrating that these preservatives are absorbed through skin and enter systemic circulation rather than remaining localized where products are applied. One particularly concerning study detected parabens in ninety-nine percent of breast cancer tissue samples analyzed, with highest concentrations found in the upper outer breast quadrant nearest the underarm where antiperspirants and deodorants are applied. While this correlation doesn’t prove causation—meaning we can’t conclude parabens cause breast cancer simply because they’re found in tumor tissue—it does raise disturbing questions about whether decades of daily exposure to estrogenic chemicals applied to areas near breast tissue might contribute to rising rates of hormonally-driven breast cancers.
Reproductive and Developmental Concerns
The potential reproductive effects of paraben exposure raise particular concern given evidence that endocrine disruptors can impact fertility, pregnancy outcomes, and child development. Studies examining paraben exposure during pregnancy found associations between maternal urinary paraben levels and various adverse outcomes including preterm birth, reduced birth weight, and altered reproductive tract development in male infants. While these associations prove concerning, the observational study design means we cannot definitively establish causation since numerous other factors also influence these outcomes. However, the consistency of findings across multiple independent studies strengthens concern that paraben exposure during the developmentally sensitive period of pregnancy may contribute to adverse outcomes even if it isn’t the sole or primary cause.
Male reproductive health represents another area of particular concern as animal studies demonstrate that paraben exposure reduces sperm count and quality, causes testicular changes, and decreases testosterone production in male rodents exposed during development and adulthood. Human studies have found correlations between urinary paraben levels and decreased sperm motility and increased DNA damage in sperm, though again these associations don’t prove causation. The declining sperm counts and male fertility observed across developed nations over recent decades have prompted extensive speculation about potential environmental contributors, with endocrine disrupting chemicals including parabens among the leading suspects given their widespread exposure and known effects on male reproductive systems in animal research.
The concern about developmental exposure to parabens centers on research showing that endocrine disrupting chemicals exert their most significant effects during periods when hormone-dependent processes guide normal development—particularly fetal development and puberty. Even brief exposures during these critical windows can cause permanent alterations in reproductive tract development, brain organization, immune system programming, and metabolic regulation that persist throughout life and potentially even affect subsequent generations through epigenetic changes passed to offspring. The ubiquity of paraben exposure means that essentially every child develops in environments where these synthetic estrogens are present, creating a massive uncontrolled experiment with outcomes that may not become fully apparent for decades.
The Industry's Paraben Defense
Despite mounting scientific concern, the cosmetic industry vigorously defends paraben safety through several arguments emphasizing the lack of definitive proof of harm rather than the emerging evidence of concern. Industry representatives accurately note that most paraben research involves cell culture studies or animal experiments at doses exceeding typical human exposure from cosmetics, with limited epidemiological data directly linking real-world paraben exposure to adverse health outcomes in humans. They emphasize that correlation doesn’t prove causation when studies find associations between paraben levels and health problems, since numerous confounding factors might explain observed relationships without parabens themselves causing harm.
The industry also points to regulatory assessments by various scientific committees that have reviewed paraben safety and concluded these preservatives remain safe for cosmetic use at the concentrations typically employed. However, these regulatory safety assessments generally occurred years or decades ago based on older research that didn’t include more recent studies identifying endocrine disruption concerns. Additionally, safety assessments typically evaluate individual parabens in isolation rather than assessing cumulative exposure from multiple products containing different paraben types plus other endocrine disrupting chemicals present in cosmetics and environment, potentially underestimating total exposure and synergistic effects.
The practical reality involves genuine scientific uncertainty about the significance of paraben exposure from cosmetics for causing serious health effects, but this uncertainty cuts both ways—it doesn’t prove safety any more than it proves harm. The precautionary principle suggests that evidence of plausible concern warrants preventive action rather than waiting for conclusive proof of harm after widespread exposure has occurred. Given that effective paraben-free preservative alternatives exist allowing manufacturers to formulate products without these controversial ingredients, the continued insistence on using parabens appears to prioritize manufacturing convenience and cost savings over consumer protection even when viable safer alternatives are available.
3. Phthalates: Reproductive Harm in Your Nail Polish
Phthalates represent a class of synthetic chemicals used primarily as plasticizers making materials soft and flexible, but in cosmetics these compounds serve multiple functions including improving product texture, enhancing fragrance longevity, and maintaining formulation stability. Common phthalates in beauty products include dibutyl phthalate in nail polish, diethyl phthalate in fragrances and personal care items, and dimethyl phthalate in hair spray and other cosmetics. These chemicals have become controversial as research reveals concerning evidence linking phthalate exposure to reproductive system abnormalities, developmental effects, and endocrine disruption with particular vulnerability during prenatal development and early childhood.
Reproductive System Impacts
The most robust scientific evidence regarding phthalate harm involves effects on male reproductive development, with extensive research demonstrating that prenatal phthalate exposure disrupts the normal development of male reproductive organs through interfering with testosterone production during the critical fetal period when reproductive tract differentiation occurs. This disruption can result in a constellation of abnormalities called phthalate syndrome in animal research, characterized by reduced anogenital distance—a marker of prenatal androgen exposure—undescended testes, hypospadias where the urethral opening forms abnormally, and later reproductive consequences including reduced sperm quality and fertility problems in adulthood.
Human epidemiological studies have confirmed associations between maternal phthalate exposure during pregnancy and reduced anogenital distance in male infants, replicating in humans one of the key markers observed in animal studies and strongly suggesting that phthalates exert similar anti-androgenic effects across species. Longer-term studies following children into adolescence find correlations between prenatal phthalate exposure and reduced testosterone levels, altered pubertal development, and reproductive tract abnormalities, though the limited number of such studies and challenges in controlling for confounding variables mean uncertainty remains about the full magnitude of human reproductive effects from early-life phthalate exposure.
The mechanisms underlying reproductive toxicity involve phthalates interfering with androgen production and signaling in developing male fetuses during the precise window when hormones guide reproductive tract differentiation. The compounds inhibit enzymes essential for testosterone synthesis and disrupt gene expression patterns normally controlled by androgens, essentially creating relative androgen deficiency during development even when overall hormone levels may measure within normal ranges. This interference during sensitive developmental windows causes permanent alterations in reproductive anatomy and function that persist regardless of whether phthalate exposure continues in later life, demonstrating how prenatal chemical exposures can program lifelong health trajectories.
The Hidden Phthalate Problem: Fragrance
One of the most insidious aspects of phthalate exposure from cosmetics involves their concealment behind the generic ingredient designation fragrance or parfum on product labels. Current regulations allow cosmetic manufacturers to list fragrance as a single ingredient without disclosing the specific chemical compounds comprising their proprietary scent formulations, ostensibly to protect trade secrets from competitors. This labeling loophole means that products listing fragrance may contain multiple phthalates and dozens of other chemicals that never appear explicitly on ingredient lists, making it impossible for consumers to identify and avoid phthalate-containing products based on label information alone.
Chemical analysis of fragranced cosmetics routinely detects phthalates not disclosed in ingredient lists, with studies finding these hidden phthalates in products ranging from perfumes and lotions to shampoos and body washes. The Campaign for Safe Cosmetics testing of popular fragrances detected phthalates in seventy-two percent of products analyzed despite none of the samples disclosing these chemicals in ingredients, demonstrating how the fragrance loophole creates widespread hidden exposure that consumers cannot avoid even with careful label reading. The quantities involved prove non-trivial with some products containing phthalate concentrations reaching several percent of total formulation weight—far from the trace contamination levels that might be reasonably dismissed as insignificant.
The fragrance issue extends beyond just phthalates to encompass numerous other potentially problematic chemicals hidden behind this single catch-all ingredient term. A typical fragrance formulation may contain fifty to three hundred different chemical compounds including synthetic musks that bioaccumulate in human tissue, volatile organic compounds that off-gas into indoor air creating respiratory exposures, and various allergens and sensitizers causing skin reactions. The complete lack of ingredient disclosure for these complex chemical mixtures prevents consumers from making informed choices about exposures they’re accepting when purchasing fragranced products, essentially forcing blind trust that manufacturers select fragrance chemicals responsibly despite no regulatory requirements to do so and substantial economic incentives to prioritize scent characteristics and cost over safety considerations.
Shop on AliExpress via link: wholesale-phthalate-free-cosmetics
Exposure Levels and Health Significance
Biomonitoring data consistently confirms ubiquitous phthalate exposure in developed nation populations, with metabolites of multiple phthalate types detected in urine samples from virtually everyone tested. Women of reproductive age show particularly high exposures compared to other demographic groups, likely reflecting greater cosmetic use and the presence of phthalates in numerous personal care products women use more frequently than men. This elevated exposure in women of reproductive age raises special concern given the evidence that prenatal phthalate exposure affects fetal development, meaning the women most heavily exposed are precisely those whose exposures carry greatest risk for the next generation.
The health significance of real-world phthalate exposure levels remains debated with industry representatives emphasizing that detected exposure levels fall below thresholds shown to cause adverse effects in animal studies, while public health advocates note that thresholds derived from animal research may not adequately protect humans—particularly for effects on developmental processes where even subtle disruptions can cause lasting impacts. Additionally, the reference doses used in safety assessments typically evaluate individual phthalates in isolation despite humans being exposed to mixtures of multiple phthalate types plus other chemicals that may act additively or synergistically to produce greater effects than any single compound alone.
The epidemiological evidence linking real-world phthalate exposures to health outcomes has strengthened substantially over the past decade as long-term studies mature and more sophisticated statistical analyses account for confounding factors. While definitive proof of causation remains elusive for most health endpoints—an inherent limitation of human observational research where we cannot ethically conduct controlled exposure experiments—the consistency of associations across multiple independent studies using different populations and methods builds confidence that observed relationships likely reflect genuine causal effects rather than statistical artifacts or confounding. The combination of strong mechanistic evidence from animal studies showing how phthalates cause reproductive harm, human biomonitoring confirming widespread exposure, and epidemiological data linking exposures to adverse outcomes creates a compelling if not absolutely conclusive case for concern.
4. Formaldehyde: Cancer-Causing Preservatives in Your Shampoo
Formaldehyde and formaldehyde-releasing preservatives represent one of the more clear-cut cases of toxic chemicals in cosmetics given that formaldehyde is definitively classified as a human carcinogen by major scientific and regulatory bodies worldwide based on extensive evidence linking inhalation exposure to nasopharyngeal cancer and possibly leukemia. Despite this unambiguous hazard classification, formaldehyde and preservatives that slowly release formaldehyde remain widely used in cosmetics including shampoos, body washes, moisturizers, and particularly keratin hair-smoothing treatments where formaldehyde concentrations can reach levels causing acute respiratory irritation during salon application.
Formaldehyde-Releasing Preservatives
While pure formaldehyde use in cosmetics has declined following increased awareness of cancer risks, formaldehyde-releasing preservatives continue appearing in numerous products where they function as antimicrobial agents preventing bacterial and fungal contamination. These preservative compounds including quaternium-15, DMDM hydantoin, imidazolidinyl urea, diazolidinyl urea, polyoxymethylene urea, sodium hydroxymethylglycinate, and 2-bromo-2-nitropropane-1,3-diol slowly break down over time releasing formaldehyde that provides the actual preservative activity. This approach allows manufacturers to avoid listing formaldehyde explicitly on ingredient labels while still deriving antimicrobial benefits from the chemical, creating a situation where consumers trying to avoid formaldehyde cannot easily identify products containing these releaser compounds without memorizing the various chemical names under which they appear.
The formaldehyde released from these preservatives accumulates in products over time as the compounds gradually break down, meaning that formaldehyde concentrations increase during shelf life potentially reaching levels above regulatory thresholds by the time consumers use products even if initial manufacturing concentrations complied with regulations. Temperature accelerates formaldehyde release from these preservatives, causing products stored in warm bathrooms or shipped during summer to accumulate higher formaldehyde levels than those kept under ideal cool conditions. This progressive release means that testing formaldehyde concentrations at manufacture provides limited assurance about actual exposures consumers experience when using products months later after storage under variable conditions.
The health concern with formaldehyde-releasing preservatives extends beyond cancer risks to include sensitization causing allergic contact dermatitis in susceptible individuals. Formaldehyde is one of the most common causes of cosmetic-related allergic skin reactions, with dermatology literature documenting hundreds of case reports of allergic contact dermatitis triggered by products containing formaldehyde or formaldehyde releasers. The prevalence of formaldehyde sensitivity in the general population is estimated around two to three percent based on patch testing studies, meaning millions of Americans experience allergic reactions to products containing these preservatives even before considering the broader population exposure to potential carcinogenic effects.
Brazilian Blowout Controversy
The keratin hair-smoothing treatments marketed under names like Brazilian Blowout became controversial when investigation revealed these products contained formaldehyde concentrations far exceeding levels considered safe despite manufacturers claiming their formulations were formaldehyde-free. The treatments work by using formaldehyde to create chemical bonds that smooth and straighten hair, but the high temperatures required during application volatilize significant quantities of formaldehyde creating acute inhalation exposure for both salon workers and customers during the multi-hour treatment process. Testing by safety regulators found formaldehyde concentrations in some keratin treatments reaching ten to twelve percent—more than twenty times above the threshold for labeling requirements and hazard warnings.
Salon workers exposed to repeated keratin treatment fumes have reported respiratory symptoms including coughing, difficulty breathing, nose and throat irritation, and headaches, with some experiencing such severe reactions they required emergency medical treatment or permanent career changes. Customer complaints included similar acute symptoms plus hair loss, scalp burns, and rashes. The controversy intensified when manufacturers continued claiming products were formaldehyde-free despite independent testing repeatedly detecting high concentrations, relying on technical arguments that formaldehyde only forms when products are heated during use rather than existing in bottles at manufacture—a distinction without meaningful difference for the actual exposures experienced by salon workers and customers.
The regulatory response to dangerous keratin treatments revealed limitations in cosmetic safety enforcement even for clear-cut cases of mislabeled products causing documented acute harm. Despite multiple states and foreign countries banning specific formulations and the Occupational Safety and Health Administration warning salons about exposure risks, many products remained available through online retailers and underground channels where determined customers could still purchase despite regulatory actions. The incident demonstrated how inadequate cosmetic regulations create situations where even egregious safety violations face limited consequences and products continue reaching consumers despite causing documented harm.
5. PFAS Forever Chemicals: The Toxins That Never Leave
PFAS—per- and polyfluoroalkyl substances—represent one of the most concerning categories of chemicals in modern cosmetics due to their extraordinary persistence in both the environment and human body, earning them the ominous designation “forever chemicals” because they resist all natural degradation processes and accumulate indefinitely wherever they’re released. These synthetic fluorinated compounds appear in cosmetics primarily to provide water-resistance, improve texture and spreadability, enhance product durability, and increase wear-time for foundations, lipsticks, mascaras, and other color cosmetics where long-lasting performance represents a key selling point. The very properties that make PFAS valuable to cosmetic manufacturers—their extreme stability and resistance to breakdown—create the fundamental problem that once these chemicals enter your body or the environment, they persist essentially forever with consequences we’re only beginning to understand.
What Makes PFAS Forever Chemicals
The molecular structure of PFAS involves chains of carbon atoms completely surrounded by fluorine atoms creating one of the strongest chemical bonds known to science. This carbon-fluorine bond proves so stable that virtually no natural biological or environmental processes can break it, meaning PFAS compounds released into ecosystems or absorbed into human bodies remain intact indefinitely rather than degrading into harmless breakdown products as most organic chemicals eventually do. The thousands of different PFAS variants share this fundamental fluorinated structure while differing in chain length and functional groups, creating a vast family of related compounds all possessing the persistent, bioaccumulative characteristics that make this chemical class so problematic.
When PFAS enter the human body through dermal absorption from cosmetics, inhalation of airborne particles, or ingestion of contaminated food and water, they distribute throughout organs and tissues where they bind to proteins and accumulate over time since the body lacks effective mechanisms to metabolize or excrete these foreign fluorinated compounds. Biomonitoring studies detect PFAS in blood serum of virtually everyone tested in developed nations, with concentrations reflecting cumulative lifetime exposure from all sources including cosmetics, food packaging, non-stick cookware, stain-resistant textiles, and contaminated drinking water. The half-lives of major PFAS compounds in human blood range from two to eight years depending on specific variant, meaning even a single exposure creates body burdens that persist for years or decades while ongoing exposures from multiple sources cause continuous accumulation throughout life.
The environmental persistence of PFAS creates contamination that spreads globally through water systems, air currents, and food chains, with these chemicals now detected in remote locations from Arctic ice to deep ocean trenches despite never being directly used in those environments. PFAS released from cosmetics wash down drains into wastewater treatment systems that cannot remove these persistent compounds, allowing them to pass through into rivers, lakes, and eventually oceans where they accumulate in marine life and cycling back to humans through seafood consumption. The agricultural use of sewage sludge as fertilizer spreads PFAS into soils where crops absorb them, creating food contamination that contributes to total human exposure alongside direct cosmetic sources. This environmental cycling means that even individuals who avoid PFAS-containing products still experience exposure from legacy contamination created by decades of unrestricted use.
Health Effects of PFAS Exposure
The health concerns associated with PFAS exposure span multiple organ systems and disease categories, with the strongest evidence linking these forever chemicals to immune suppression, thyroid disease, elevated cholesterol, pregnancy complications, and increased cancer risk particularly for kidney and testicular cancers. Epidemiological studies of communities with documented high PFAS exposure through contaminated drinking water have found elevated rates of multiple health problems compared to unexposed populations, though establishing causation proves challenging given the complexity of multi-factorial diseases and the ubiquity of low-level PFAS exposure in modern populations making unexposed control groups difficult to identify.
The immune system effects of PFAS prove particularly concerning as research demonstrates that exposure reduces antibody responses to vaccinations in both children and adults, suggesting these chemicals compromise the immune system’s ability to generate protective immunity against infectious diseases. Studies of children with measured PFAS blood levels found that higher exposures correlated with reduced vaccine antibody production, requiring some children to receive additional vaccine doses to achieve protective immunity. In the context of global health challenges where vaccine effectiveness proves crucial for disease control, the discovery that ubiquitous chemical exposures may be undermining vaccine protection raises serious public health concerns extending far beyond individual health to population-level disease vulnerability.
Thyroid disruption represents another well-documented PFAS health effect, with research showing these chemicals interfere with thyroid hormone production and metabolism causing alterations in hormone levels that can affect metabolism, energy, mood, and development. Pregnant women with elevated PFAS exposure show increased risk of thyroid problems during pregnancy—a particularly vulnerable period when thyroid hormones play critical roles in fetal brain development. Children exposed to higher PFAS levels show increased rates of thyroid disease, with effects potentially extending throughout life since the thyroid regulates numerous body functions and thyroid disorders often require lifelong medication to manage.
The cancer risks associated with PFAS gained significant attention when research in communities with extremely high exposures through contaminated water supplies documented elevated rates of kidney and testicular cancer, with subsequent laboratory studies identifying mechanisms by which these chemicals could promote tumor development. While the cancer associations observed in highly-exposed populations may not translate directly to lower exposures experienced by the general public through cosmetics and other consumer products, the mechanistic evidence that PFAS can interfere with cellular processes regulating growth and division raises concern that even lower-level chronic exposures may contribute to cancer risk over decades of accumulation.
Shop on AliExpress via link: wholesale-pfas-free-makeup
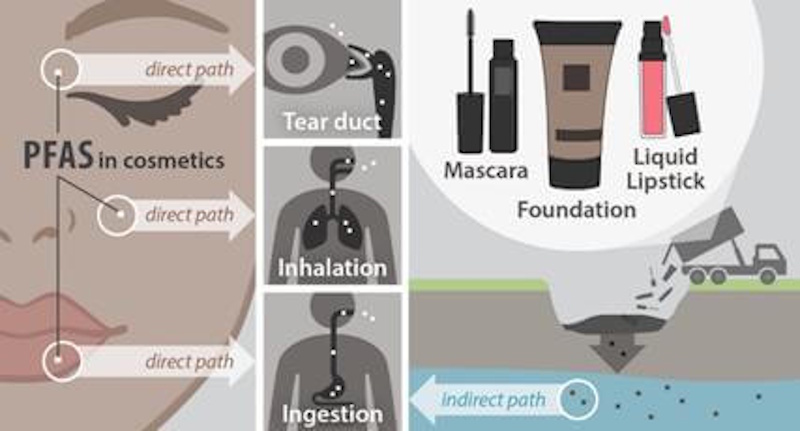
PFAS in Cosmetics: Hidden Contamination
The presence of PFAS in cosmetics occurs through both intentional addition where manufacturers include these chemicals specifically for their performance benefits and through contamination where PFAS appear as impurities in ingredients or migrate from packaging materials. Intentional PFAS use appears most commonly in products marketed with claims about long-lasting wear, water-resistance, or transfer-resistance—characteristics these chemicals effectively provide through their unique properties. Testing by environmental health organizations has detected PFAS in roughly half of makeup products analyzed, with particularly high detection rates in waterproof mascara, liquid lipstick, foundation, and concealer categories where performance claims often depend on PFAS inclusion.
The challenge of identifying PFAS-containing cosmetics proves substantial because current labeling regulations don’t require disclosure of these chemicals, and the thousands of PFAS variants appear under diverse chemical names that consumers cannot possibly memorize or recognize. Some PFAS used in cosmetics include ingredients with names like perfluorohexane, perfluorodecalin, perfluoroperhydrophenanthrene, and polytef—technical terms that reveal nothing to average consumers about these being the forever chemicals linked to serious health concerns. Manufacturer claims about PFAS-free formulations sometimes prove unreliable as testing reveals PFAS presence in products marketed as free of these chemicals, suggesting either inadvertent contamination or misleading marketing.
The regulatory response to PFAS in cosmetics remains inadequate despite growing awareness of these chemicals’ problems, with most jurisdictions lacking specific restrictions on PFAS use in beauty products. Some progressive states in the US have passed or proposed legislation restricting PFAS in cosmetics, but federal regulations remain absent and manufacturers continue using these chemicals freely in most products. International restrictions prove slightly more robust with the European Union considering proposals to ban PFAS across numerous product categories including cosmetics, though implementation faces industry opposition and concerns about enforcement complexity given the thousands of PFAS variants that would require monitoring.
6. Heavy Metals: Lead and Arsenic in Your Lipstick
Heavy metal contamination in cosmetics represents one of the most disturbing ingredient problems because these toxic elements appear as unintentional contaminants in color pigments and mineral-derived ingredients rather than being deliberately added, creating situations where even manufacturers may not know their products contain lead, arsenic, cadmium, or other metals unless specific testing is conducted. The health risks of heavy metal exposure prove well-established through decades of research documenting neurotoxicity from lead, cancer risks from arsenic and cadmium, and organ damage from chronic exposure to various metallic elements. Yet cosmetic regulations in most countries establish no maximum contamination levels for heavy metals in beauty products, no mandatory testing requirements before products reach market, and minimal post-market surveillance to identify problematic products after they’re already being used by millions of consumers.
Lead in Lipstick: The Persistent Problem
Lead contamination in lipstick gained widespread attention when testing by the FDA and consumer advocacy organizations detected this neurotoxic heavy metal in virtually every lipstick tested, with concentrations ranging from trace amounts to several parts per million depending on color shade and brand. The lead doesn’t get added intentionally but rather enters products as a contaminant in color pigments derived from mineral sources where lead naturally co-occurs with the desired coloring agents. Darker and more vibrant lip colors typically show higher lead content because achieving these shades requires greater pigment concentrations, inadvertently increasing the lead contamination proportionally.
The health concern with lead in lipstick involves cumulative neurotoxic effects from chronic low-level exposure, with lead causing cognitive impairment, developmental delays in children, cardiovascular effects, and reproductive harm particularly when exposure occurs during pregnancy affecting fetal brain development. No safe exposure level exists for lead according to leading health authorities, meaning any exposure carries some risk with effects occurring across the full range of blood lead concentrations measured in human populations including levels previously considered benign. Women using lipstick daily ingest significant quantities of product over time through the unavoidable transfer from lips to mouth during eating, drinking, and normal lip moistening, creating chronic oral exposure to whatever contaminants exist in formulations.
The regulatory response to lead in lipstick reveals fundamental problems with cosmetic safety oversight, as the FDA conducted testing demonstrating widespread contamination but established no maximum allowable lead levels, issued no recalls, and took no enforcement action despite knowing that products consumers use daily contain a substance with no safe exposure threshold. The agency’s position that detected lead levels don’t pose safety concerns conflicts with the scientific consensus that no safe lead level exists, creating confusion about whether lead-contaminated lipsticks actually present hazards or merely theoretical concerns that can be safely ignored. Other countries including Canada have established specific limits on lead content in cosmetics following similar testing revelations, demonstrating that regulatory action is possible when authorities prioritize consumer protection over industry convenience.
Arsenic, Cadmium, and Other Metal Contaminants
Beyond lead, testing has detected arsenic, cadmium, chromium, nickel, and other potentially harmful metals in various cosmetic products at levels raising concern among toxicologists and public health advocates. Arsenic—a known human carcinogen linked to skin, lung, and bladder cancer—appears in some cosmetics as a contaminant in mineral ingredients, with the inorganic arsenic forms found in cosmetics being the most toxic variants of this element. Cadmium contamination occurs in pigments providing yellow, orange, and red colors, with this heavy metal causing kidney damage and bone problems through chronic exposure while also being classified as a probable human carcinogen.
The sources of metal contamination include mineral pigments mined from deposits containing various metallic elements as natural contaminants, with purification processes removing some but not all contaminating metals before pigments get sold to cosmetic manufacturers. The economic incentives favor minimal purification since extensive refining increases costs while current regulations don’t penalize contamination, creating situations where suppliers provide the cheapest pigments meeting basic color specifications regardless of heavy metal content. Manufacturers purchasing these pigments often don’t conduct contamination testing, instead relying on supplier assurances and the absence of regulatory requirements to justify skipping expensive testing that might identify problems requiring reformulation.
The cumulative exposure from multiple cosmetic products containing trace heavy metal contamination creates body burdens that accumulate over time since elimination of these metals occurs slowly if at all depending on the specific element and chemical form. Lead and cadmium in particular bioaccumulate in bone and organs where they remain for years or decades continuously exerting toxic effects even after external exposure ceases. The combination of persistence, cumulative effects, and no safe exposure thresholds means that even low-level contamination in individual products contributes to total lifetime metal burdens that may eventually reach levels causing measurable health effects.
Mineral Makeup and Metal Contamination
Mineral makeup products marketed with natural and clean beauty claims ironically often contain higher heavy metal contamination than conventional cosmetics because mineral-derived pigments represent the primary contamination source. Titanium dioxide and zinc oxide used in mineral sunscreens and foundations can contain trace arsenic, lead, and other metallic elements depending on mining source and purification rigor. Iron oxides providing color in mineral makeup formulations similarly may contain heavy metal contaminants if sourced from deposits with contamination or inadequately purified before cosmetic use. The natural origins of these ingredients don’t guarantee purity, and absent mandatory testing requirements, consumers have no reliable way to distinguish carefully purified mineral ingredients from contaminated versions.
The marketing of mineral makeup as a safer natural alternative to synthetic cosmetics creates ironic situations where products purchased specifically to avoid chemical exposure potentially deliver greater heavy metal exposure than conventional formulations using synthetic pigments without metal contamination. This highlights the fundamental problem that natural doesn’t equal safe, and clean beauty marketing claims provide no assurance of actual ingredient purity or safety absent verification through independent testing. Truly clean mineral makeup requires sourcing pigments from uncontaminated deposits or implementing rigorous purification processes removing metallic contaminants, steps that increase costs and require commitment to safety that marketing claims alone don’t guarantee manufacturers are actually undertaking.
7. Synthetic Fragrances: Toxic Cocktails Behind One Word
Synthetic fragrances represent perhaps the most deceptive ingredient category in cosmetics through the regulatory loophole allowing manufacturers to conceal complex mixtures of dozens or even hundreds of individual chemicals behind the single catch-all ingredient designation “fragrance” or “parfum” on product labels. This disclosure exemption ostensibly protects proprietary scent formulations from competitors but functionally prevents consumers from knowing which specific chemicals they’re exposing themselves to through fragranced products, making it impossible to avoid particular substances even with careful label reading. The hidden chemicals behind fragrance listings include numerous compounds associated with allergies, hormone disruption, respiratory problems, and other health concerns, with no requirement that manufacturers prove the safety of their undisclosed fragrance mixtures or report adverse reactions consumers experience from these concealed ingredients.
The Fragrance Loophole
The regulatory allowance for non-disclosure of fragrance ingredients originated from legitimate business concerns about protecting proprietary scent formulations that represent significant investment in perfume development and provide competitive advantages through distinctive scents. However, this trade secret protection has expanded far beyond its original justification to encompass hiding chemicals with no legitimate intellectual property value behind generic fragrance designations. Phthalates used as fragrance solvents and fixatives provide no unique scent characteristics but routinely hide behind fragrance labels despite being reproductive toxicants that consumers might reasonably want to avoid. Similarly, numerous synthetic musks, volatile organic compounds, and other problematic chemicals hide in fragrance mixtures without disclosure despite having nothing to do with protecting proprietary scent formulas.
The International Fragrance Association maintains a list of roughly four thousand ingredients used in fragrance formulations, providing some indication of the chemical complexity hiding behind that single ingredient word on product labels. Many of these fragrance chemicals have never undergone thorough safety testing for chronic exposure effects, reproductive toxicity, or potential to disrupt endocrine systems despite being included in products people use daily. The fragrance industry’s internal safety assessments lack independence and transparency compared to regulatory safety evaluations, creating situations where chemical safety determinations are made by the very industry profiting from unrestricted chemical use rather than independent scientists without financial conflicts of interest.
The European Union requires disclosure of twenty-six fragrance allergens when present above specific threshold concentrations, providing consumers some information about particularly problematic fragrance chemicals even when complete ingredient lists remain concealed. However, this limited disclosure still hides hundreds of other fragrance ingredients that might concern consumers for reasons beyond allergy potential, and the threshold concentrations mean that products can contain these allergens without disclosure if kept below specified levels. American regulations require no such allergen disclosure, leaving consumers completely ignorant about even the most common fragrance allergens in products they purchase unless manufacturers voluntarily provide information beyond minimal legal requirements.
Shop on AliExpress via link: wholesale-fragrance-free-beauty-products
Health Impacts of Fragrance Exposure
Allergic reactions to fragrances represent the most immediate and obvious health concern, with dermatologists identifying fragrance as one of the leading causes of cosmetic-related contact dermatitis. Fragrance allergies can develop at any age after sufficient exposure to sensitizing chemicals, with some individuals becoming so sensitized that even trace fragrance exposure triggers severe reactions. The prevalence of fragrance allergy in the general population is estimated around two to three percent based on patch testing studies, though the real rate may be higher since many people with fragrance sensitivities never undergo formal allergy testing and simply avoid fragranced products after noticing reactions.
Respiratory effects from inhaled fragrance chemicals concern pulmonologists and environmental health researchers as studies document that volatile fragrance components trigger asthma attacks, aggravate existing respiratory conditions, and cause symptoms including cough, wheezing, and shortness of breath even in individuals without diagnosed asthma. The mechanism involves fragrance chemicals irritating airway tissues and triggering inflammatory responses that constrict breathing passages, with some fragrance components also identified as respiratory sensitizers that can cause development of new asthma or chemical sensitivity through repeated exposure.
The endocrine disrupting properties of various fragrance chemicals raise concerns that extend beyond immediate symptoms to potential long-term effects on hormone-dependent processes throughout the body. As discussed earlier, phthalates commonly used in fragrances interfere with reproductive hormone systems. Synthetic musks accumulate in human tissues and breast milk while demonstrating estrogenic activity in laboratory studies. Various other fragrance components show hormone-disrupting properties in research despite being included in products without disclosure or safety assessment of their endocrine effects.
Fragrance-Free Isn't Always Fragrance-Free
The complexity of navigating fragrance safety extends to confusion about products labeled fragrance-free or unscented which consumers might reasonably assume contain no fragrance chemicals. However, these terms lack regulatory definitions allowing manufacturers to use them inconsistently or misleadingly. Some fragrance-free products genuinely contain no added fragrance ingredients while others include fragrance chemicals specifically chosen for weak scents or masking agents that hide the natural odors of other ingredients without adding noticeable fragrance, technically allowing products to be labeled fragrance-free despite containing fragrance chemicals.
Unscented products particularly confuse consumers as this term often means products contain masking fragrances that cover the natural scent of ingredients rather than containing no fragrance at all. A product can smell relatively neutral while actually containing substantial fragrance chemicals chosen to mask rather than create noticeable scent. For individuals with fragrance sensitivities or those trying to minimize chemical exposures, the presence of masking fragrances defeats the purpose of selecting unscented products, yet current labeling rules provide no reliable way to distinguish genuinely fragrance-free formulations from those using masking agents.
The most reliable approach to avoiding fragrance involves seeking products that both state fragrance-free and specifically list all ingredients including any used for masking purposes rather than hiding them behind fragrance exemptions. Some clean beauty brands provide complete ingredient transparency including disclosure of any aroma chemicals used, but such transparency remains voluntary and uncommon leaving consumers largely unable to make truly informed choices about fragrance exposure from the products they purchase.
8. Chemical Sunscreens: Protection or Poison
Chemical sunscreens create a particularly complex ingredient safety challenge because while sun protection unquestionably reduces skin cancer risk making sunscreen use medically beneficial, growing evidence suggests that several chemical UV filters commonly used in sunscreen formulations may themselves present health risks through hormone disruption, allergic reactions, and environmental damage. This creates genuine trade-offs where the undeniable benefits of sun protection must be weighed against potential harms from specific sunscreen ingredients, with the optimal resolution involving using mineral sunscreens or carefully selected chemical formulations rather than abandoning sun protection entirely or uncritically accepting any sunscreen as safe simply because it protects against UV damage.
Oxybenzone and Octinoxate Concerns
Oxybenzone represents the most controversial chemical sunscreen ingredient due to extensive research demonstrating this UV filter acts as an endocrine disruptor with estrogenic, anti-androgenic, and thyroid-disrupting properties in laboratory studies and documented bioaccumulation in human tissues. Biomonitoring studies detect oxybenzone in the urine of nearly all Americans tested, confirming that skin absorption from sunscreen application allows systemic exposure to this hormone-disrupting chemical rather than localized skin surface effects. Research has found correlations between urinary oxybenzone levels and various reproductive effects including altered hormone levels, endometriosis, and reduced male fertility, though as with other ingredient research, establishing definitive causation from observational studies remains challenging.
The mechanism of oxybenzone’s endocrine disruption involves binding to estrogen and androgen receptors where it can either mimic or block natural hormone signaling depending on context. Animal studies demonstrate that oxybenzone exposure during development causes reproductive tract abnormalities, altered pubertal timing, and persistent reproductive effects extending into adulthood even after exposure ceases. Pregnant women show particularly high oxybenzone exposure through increased sunscreen use during beach vacations and summer activities, creating concern that fetal exposure during development may contribute to reproductive programming effects similar to those observed in animal research.
Octinoxate (also called octyl methoxycinnamate) shares similar endocrine disrupting properties with oxybenzone while also raising environmental concerns through its toxicity to coral reefs and aquatic life. Research demonstrates octinoxate causes feminization of male fish through estrogenic effects and contributes to coral bleaching events that destroy reef ecosystems. Several jurisdictions including Hawaii, Key West, and the US Virgin Islands have banned both oxybenzone and octinoxate from sunscreens due to coral damage despite the chemicals remaining legal in most locations due to inadequate marine environmental regulations.
The Mineral Sunscreen Alternative
Mineral sunscreens using zinc oxide or titanium dioxide as UV filters provide effective sun protection without the endocrine disruption and environmental concerns associated with chemical filters, making them the preferred option for consumers prioritizing ingredient safety. These mineral ingredients work through physical mechanisms reflecting and scattering UV radiation rather than absorbing it through chemical reactions like oxybenzone and octinoxate, preventing UV penetration into skin without requiring absorption of active ingredients into systemic circulation. Mineral sunscreens have demonstrated environmental safety superior to chemical alternatives, with no evidence of coral reef damage or aquatic toxicity at concentrations released from recreational swimming.
The drawbacks of mineral sunscreens involve aesthetic characteristics including white cast where zinc oxide and titanium dioxide leave visible white residue on skin after application, particularly problematic for people with darker skin tones where the white cast proves especially noticeable and cosmetically unacceptable. Texture issues where mineral sunscreens feel heavier and greasier than lightweight chemical formulations also limit acceptance particularly among consumers accustomed to elegant chemical sunscreen textures. The cosmetic challenges of mineral formulations have driven development of tinted mineral sunscreens that minimize white cast and micronized particles that improve texture, though some consumers remain skeptical of nanoparticle forms despite lack of evidence suggesting nano zinc oxide or titanium dioxide penetrate healthy skin or cause harm.
The safety profile of mineral sunscreens proves superior to chemical alternatives with decades of use demonstrating no serious adverse effects beyond occasional mild skin irritation in sensitive individuals. Neither zinc oxide nor titanium dioxide shows endocrine disrupting properties, bioaccumulation concerns, or the systemic absorption that enables chemical sunscreen ingredients to reach reproductive organs and developing fetuses. For parents protecting children—particularly infants and toddlers whose developing systems prove most vulnerable to endocrine disruption—mineral sunscreens represent the clear safer choice even accounting for the cosmetic compromises these formulations require.
Newer Chemical Filters: Safer Alternatives?
The limitations of both conventional chemical sunscreens with endocrine disrupting properties and mineral formulations with cosmetic challenges have driven development of newer chemical UV filters designed to provide sun protection without the hormone disruption issues of older ingredients. Compounds like avobenzone, homosalate, octisalate, and octocrylene represent chemical filters generally considered safer than oxybenzone and octinoxate based on current evidence, though long-term safety data remains limited compared to the extensively studied problematic filters. The trade-off involves accepting some uncertainty about novel ingredients in exchange for avoiding chemicals with documented concerns while still achieving the elegant cosmetic properties that make chemical sunscreens more appealing than mineral alternatives for many consumers.
Avobenzone serves as the most widely used UVA filter in chemical sunscreens marketed in the United States, providing broad-spectrum protection that mineral filters previously dominated. Current evidence suggests avobenzone doesn’t show the strong endocrine disrupting effects documented for oxybenzone, though some studies have identified weak estrogenic activity and concerns about photostability where avobenzone degrades under sun exposure creating potentially problematic breakdown products. Formulation with photostabilizers mitigates degradation concerns, but the need for additional ingredients to stabilize primary UV filters adds complexity to safety assessment of complete formulations versus individual ingredients.
The regulatory approval process for new sunscreen ingredients in the United States has proven extremely slow and cumbersome, with ingredients widely used in European and Asian sunscreens remaining unavailable in American products due to regulatory barriers preventing approval despite extensive international safety data. This means American consumers have access to fewer sunscreen options and particularly lack modern chemical filters that might offer improved safety profiles compared to older ingredients while maintaining the cosmetic elegance that drives consumer acceptance. Reform of sunscreen approval processes could expand access to potentially safer alternatives while competition from improved chemical filters might reduce reliance on problematic ingredients like oxybenzone by providing manufacturers viable alternatives that consumers actually want to use.
9. How to Read Ingredient Labels Like an Expert
Becoming proficient at evaluating cosmetic ingredient safety requires developing skills to interpret complex chemical names on product labels, recognize various forms under which problematic ingredients appear, understand ingredient order and concentration implications, and navigate the numerous misleading marketing claims that create false impressions of safety or purity. While perfect knowledge of every cosmetic ingredient proves impossible given the thousands of chemicals used across product categories, focusing on the highest-priority problematic ingredients enables meaningful risk reduction even without comprehensive chemistry expertise. Combined with strategic use of ingredient-checking resources and databases, informed consumers can make dramatically safer product choices without needing formal scientific training in cosmetic chemistry or toxicology.
Decoding Chemical Names
The technical chemical names appearing on cosmetic labels intimidate most consumers who reasonably feel overwhelmed by terms like methylchloroisothiazolinone, tetrasodium EDTA, or phenoxyethanol without obvious meaning to laypeople lacking chemistry backgrounds. However, learning to recognize common word elements and patterns enables identification of ingredient categories without memorizing every individual chemical name. The suffix -paraben identifies all paraben preservatives regardless of the prefix describing which specific paraben variant, allowing recognition of methylparaben, propylparaben, butylparaben, and others through the shared word ending. Similarly, phthalates typically include “phthalate” in their names like dibutyl phthalate or diethyl phthalate, while formaldehyde-releasing preservatives often contain distinctive elements like “quaternium” or include formaldehyde-related terms in their names.
The International Nomenclature of Cosmetic Ingredients system establishes standardized names meant to provide consistency across products and countries, but these technical names frequently differ from common names or chemical names used in scientific literature, creating confusion when trying to match ingredient list terms with problematic chemicals identified in research or advocacy materials. For instance, the INCI name for retinol is retinol, but the chemical class is called retinoids in scientific literature and vitamin A in marketing materials—subtle variations that can cause consumers to miss connections between ingredients and their broader chemical families. Learning key synonyms and alternative names for priority ingredients helps navigate this terminology complexity.
The ingredient order on labels provides information about concentration since regulations require listing ingredients in descending order of predominance, though the cutoff threshold for this ordering requirement varies by jurisdiction. Ingredients present above one percent must be listed in order of concentration in most regulations, while those below one percent can be listed in any order following the properly-ordered major ingredients. This means that problematic ingredients appearing early in ingredient lists represent more significant exposure than those at the end, though even trace amounts of particular chemicals may concern consumers aiming to avoid specific substances entirely.
Recognizing Greenwashing and Misleading Claims
The explosion of consumer interest in clean beauty and ingredient safety has generated corresponding explosion of marketing claims designed to create impressions of product purity and safety regardless of whether formulations actually avoid problematic ingredients. Terms like natural, clean, green, eco-friendly, non-toxic, pure, and countless other aspirational descriptors appear on products without legal definitions requiring specific formulation standards or ingredient restrictions, allowing manufacturers to apply these labels based on minimal changes like removing one controversial ingredient while retaining numerous others. Understanding which claims have regulatory meaning versus which represent pure marketing fluff helps consumers avoid paying premium prices for products whose actual safety profiles don’t meaningfully differ from conventional alternatives costing substantially less.
Organic certification in cosmetics carries different meanings depending on which standard applies, with variations ranging from requiring seventy percent organic content of agricultural ingredients (not total formula since water and minerals don’t count) to requiring ninety-five percent or even one hundred percent organic in the strictest standards. Products labeled “made with organic ingredients” typically contain lower organic percentages than those carrying actual organic certification, exploiting consumer confusion about these distinctions to imply superior purity through highlighting organic ingredients in marketing without formulation meeting stricter organic certification standards. Moreover, organic certification addresses agricultural practices and pesticide residues rather than overall ingredient safety, meaning organic products can still contain preservatives, fragrances, and other synthetic ingredients that concern consumers focused on chemical safety versus agricultural methods.
Cruelty-free and vegan claims address animal welfare rather than ingredient safety, with products carrying these designations potentially containing any problematic synthetic chemicals as long as no animal testing occurred during development and no animal-derived ingredients appear in formulations. While animal welfare represents legitimate ethical concern, consumers conflating these claims with overall safety may choose products based on animal testing status while inadvertently accepting formulations with numerous harmful ingredients since cruelty-free and vegan designations provide no assurance of human safety. The marketing often deliberately encourages this conflation through presenting animal welfare claims alongside aspirational imagery suggesting purity and safety, creating impressions that extend beyond what the specific claims actually verify.
The most reliable approach involves ignoring marketing claims entirely and directly evaluating ingredient lists against resources like the Environmental Working Group’s Skin Deep database, Think Dirty app, or similar ingredient-checking tools that assess ingredients based on scientific evidence of health concerns rather than marketing terminology. While no rating system proves perfect and different organizations emphasize different safety priorities creating inconsistent ratings across platforms, cross-referencing multiple sources provides more reliable information than trusting manufacturer marketing or assuming that regulatory approval guarantees safety given the inadequate oversight discussed throughout this guide.
10. Building Your Safe Beauty Routine
Transitioning from conventional beauty products containing problematic ingredients to a safer routine optimized for minimizing exposure requires balancing multiple considerations including ingredient safety priorities, product performance and efficacy, aesthetic characteristics and sensory experience, budget constraints and cost-effectiveness, and practical availability of suitable alternatives. The optimal approach varies dramatically across individuals depending on personal priorities, risk tolerance, skin type and concerns, current product dependency, and willingness to compromise on certain performance characteristics in exchange for safer ingredient profiles. Rather than prescribing one-size-fits-all solutions, effective guidance involves frameworks for making informed personal choices that align ingredient safety improvements with individual circumstances and priorities.
Prioritizing Product Replacement
Most people’s beauty routines include numerous products making simultaneous replacement financially impractical and psychologically overwhelming while also making it difficult to identify which specific products cause problems if adverse reactions occur during transition. Sequential replacement starting with highest-priority products enables managing both costs and change complexity while focusing initial efforts where they deliver maximum safety improvements. Several factors help prioritize which products to replace first versus which can wait until current items are exhausted or until after verifying that initial transitions proceed successfully.
Products used most frequently and in largest quantities create greatest exposure making them highest replacement priorities—daily moisturizers, foundations applied to large facial areas, and body lotions used over extensive skin surface deliver more significant chemical exposure than occasional products like nail polish or hair masks used weekly. Items applied to areas with high absorption like underarms or near mucous membranes warrant priority given that thin or damaged skin and mucous membranes allow greater chemical penetration than intact skin on less sensitive body areas. Products with longer skin contact time like overnight creams create more exposure than rinse-off items briefly applied then removed. Finally, formulations containing multiple priority ingredients of concern justify earlier replacement than products with isolated minor issues.
The category prioritization might proceed by addressing all leave-on products applied to large areas daily—moisturizers, sunscreens, foundations—before concerning oneself with occasional-use items like masks or treatments. Within each category, further prioritization might rank products by number and severity of problematic ingredients they contain, replacing worst offenders first while tolerating slightly questionable items until suitable alternatives are identified and tested. This systematic approach prevents both decision paralysis from overwhelming complexity and budgetary shock from attempting to replace everything simultaneously.
Evaluating Alternative Products
Identifying truly safer alternatives requires more diligence than simply choosing products marketed with clean beauty claims or sold by brands positioning themselves as natural or green. As discussed regarding greenwashing, marketing language often misleads with aspirational terms that don’t correlate with actual formulation safety since these descriptors lack regulatory definitions or meaningful standards. Effective product evaluation involves examining actual ingredient lists and verifying claims through independent resources rather than accepting manufacturer marketing at face value.
The ingredient-checking process should focus first on confirming absence of highest-priority ingredients to avoid—parabens, phthalates, formaldehyde and formaldehyde-releasing preservatives, PFAS, oxybenzone, and synthetic fragrances concealing undisclosed chemicals. Products passing this initial screen merit secondary evaluation examining other ingredients for any additional concerns specific to individual sensitivities or broader safety considerations. Cross-referencing ingredients against multiple evaluation resources helps identify differences in how various organizations assess the same chemicals, enabling informed decisions about which concerns warrant action versus which represent marginal issues different experts disagree about.
Beyond ingredient safety, product efficacy and sensory characteristics significantly influence long-term sustainability of safer routines since consumers rarely maintain use of products they find unpleasant or ineffective regardless of superior safety profiles. Reading reviews from people with similar skin types and concerns provides insights into whether alternatives perform adequately for intended purposes, while sample sizes enable personal testing before committing to full-size purchases of expensive items. Some trial and error proves inevitable given individual variation in skin chemistry and preferences, but research and sampling reduce the financial waste and frustration from purchasing numerous unsuitable products.
The DIY Beauty Option
Making your own beauty products from simple ingredients represents an approach guaranteeing complete ingredient control and transparency since you personally source and combine everything in formulations, eliminating uncertainty about undisclosed chemicals or contamination. Simple DIY recipes using plant oils, butters, essential oils, and basic food-grade ingredients can replicate functionality of many commercial products at significantly lower costs while providing sensory satisfaction from creating custom formulations tailored precisely to personal preferences. However, DIY beauty also presents challenges including time investment in making products, limited shelf life without commercial preservative systems, contamination risks from non-sterile home preparation, and potential for formulation errors causing skin irritation or inadequate preservation leading to microbial growth.
The most practical DIY approach focuses on simple formulations using inherently stable ingredients requiring minimal preservation rather than attempting to replicate complex commercial products like emulsions and water-based formulations that demand sophisticated preservation to remain safe during extended storage. Oil-based products like body oils, cleansing balms, and lip balms use ingredients naturally resistant to microbial growth requiring no additional preservation while providing effective functions that commercial products serve. Simple scrubs combining oils with sugar or salt offer exfoliation without preservative concerns since brief use-time prevents microbial issues even with water exposure during application.
For formulations requiring water and thus preservation, either limit batch sizes to quantities used quickly before contamination develops, or invest in learning proper preservative selection and use from reputable DIY beauty resources rather than attempting water-based formulations without adequate preservation knowledge. Improperly preserved DIY products can harbor dangerous bacterial or fungal growth causing serious infections, while under-preserved products may appear and smell fine while harboring pathogens at levels sufficient to cause harm. The enthusiastic DIY beauty community online provides extensive information but varies widely in scientific rigor, requiring critical evaluation of sources to distinguish reliable guidance from dangerous misinformation.
Conclusion: Your Health Deserves Better Than Toxic Beauty
The journey from unconscious acceptance of whatever cosmetic ingredients manufacturers choose to include to informed active avoidance of chemicals with documented health concerns represents more than simple product selection—it reflects fundamental shifts in understanding corporate ethics, regulatory adequacy, and personal agency regarding chemical exposures that modern life otherwise imposes without meaningful consent. The disturbing discoveries about toxic ingredients in daily-use beauty products reveal broader truths about how inadequately consumer protection systems function when industry interests consistently outweigh public health concerns in regulatory and political processes. While we cannot eliminate all chemical exposures from modern existence, we can identify and reduce unnecessary exposures from products chosen deliberately for purposes that should enhance wellbeing rather than compromise it.
Your face, your skin, your body deserve better than serving as uncontrolled experiments testing long-term effects of chemical combinations that have never been adequately studied for safety but get marketed anyway because regulations require so little from manufacturers prioritizing profits over protection. The beauty industry deserves accountability for formulation choices made based on cost and performance convenience rather than health protection when safer alternatives exist but require slightly higher expense or technical adjustment. Regulatory systems deserve fundamental reform closing massive gaps that allow toxic chemicals in products people use daily despite international consensus that these substances present unacceptable risks.
But until those systemic changes occur—and they will only occur through sustained pressure from informed consumers demanding better and refusing to purchase products containing ingredients they recognize as problematic—you must protect yourself through knowledge, strategic product selection, and conscious choices about which exposures to accept versus which you can effectively minimize. Every purchasing decision represents a vote about which formulations deserve market success, with collective consumer choices ultimately proving more powerful than individual regulatory actions in driving industry reformulation toward safer ingredients when companies recognize that buyers increasingly avoid problematic chemicals.
The investment of time and effort required to develop ingredient literacy and rebuild your beauty routine with safer alternatives pays returns through reduced lifetime exposure to chemicals linked to cancer, hormone disruption, reproductive harm, and numerous other serious health effects. The initial learning curve proves steep as you familiarize yourself with ingredient names, evaluation resources, and safer brand options, but competence develops quickly through repeated practice until reading ingredient lists and identifying concerns becomes automatic rather than requiring conscious effort and label-checking app consultations. The emotional journey from betrayal and anger about toxic ingredients in products you trusted to empowerment through informed choices and engagement with companies producing genuinely safer alternatives provides satisfaction beyond physical health improvements.
Different readers will take different lessons from this comprehensive exploration of cosmetic ingredient problems. Some will feel motivated to immediately discard all conventional products and completely rebuild their routines with exhaustively vetted safe alternatives. Others will focus on eliminating highest-priority problematic ingredients while accepting that perfect purity remains impossible and pragmatic harm reduction proves more achievable than zero exposure to all concerning chemicals. Still others might read this information and consciously decide they accept the risks associated with conventional products in exchange for performance, convenience, or cost characteristics those formulations provide. All represent valid personal choices when made from informed understanding rather than unconscious acceptance of whatever manufacturers decide to include.
The question facing every consumer reading these revelations involves not whether to achieve perfect safety—an impossible standard given ubiquitous chemical exposures from environment and products beyond personal control—but rather how much effort to invest in reducing unnecessary exposures from beauty products chosen deliberately and paid for voluntarily. The answer proves deeply personal depending on individual risk tolerance, health priorities, trust in incremental safer approaches versus absolutist avoidance, and countless other factors shaping how different people navigate uncertain risks in complex world where perfect information and perfect safety remain forever elusive goals rather than achievable realities.
Your beauty routine deserves to actually serve you rather than exposing you to chemicals that may undermine the health you’re attempting to protect through self-care practices including skincare and cosmetic use. The industry deserves to compete on formulation safety as a key differentiator rather than uniformly accepting minimal regulatory standards while focusing differentiation on packaging aesthetics and marketing narratives. Consumers deserve honesty about ingredient safety rather than reassuring platitudes from companies whose financial interests directly conflict with transparency about formulation problems.
The path forward requires changes at multiple levels—individual consumers educating themselves and making informed choices, companies reformulating away from problematic ingredients and embracing transparency, regulators closing massive gaps in cosmetic safety oversight, and researchers continuing to investigate health effects of ubiquitous chemical exposures to inform better policies. Progress will prove frustratingly slow given entrenched interests and political realities, but every individual choosing safer alternatives contributes to market shifts that ultimately drive reformulation even absent regulatory requirements.
Your face—the first thing people see and a central part of identity and self-expression—deserves products that genuinely care for it rather than cosmetics that beautify superficially while potentially causing internal harm invisible until damage accumulates across years or decades. Your body—the only one you have and the vehicle enabling everything you experience and accomplish throughout life—deserves protection from unnecessary chemical exposures that provide no genuine benefit while creating risks you never consciously accepted. Your choices matter both for personal health protection and collective influence driving industry change through consumer pressure where regulatory action fails.
Start today with whatever level of engagement feels manageable—perhaps replacing a single product with a safer alternative, or reading ingredient lists and researching one problematic ingredient to begin building knowledge, or simply sharing this information with others who deserve to know what they’re accepting unknowingly every time they apply conventional cosmetics. Every step toward informed choices represents progress away from blind trust and toward active agency regarding chemical exposures that modern beauty routines should never require accepting unconsciously.
Frequently Asked Questions
Question 1:
What are the most dangerous chemicals commonly found in beauty products?
Answer 1:
The most hazardous beauty chemicals with strongest evidence of serious health risks include parabens functioning as endocrine disruptors that mimic estrogen in the body and accumulate in breast tissue, phthalates linked to reproductive system abnormalities and developmental harm particularly when exposure occurs during pregnancy, formaldehyde and formaldehyde-releasing preservatives classified as human carcinogens by international health authorities based on extensive evidence linking inhalation exposure to nasopharyngeal cancer and possibly leukemia, PFAS forever chemicals that persist indefinitely in human organs and environment while contributing to immune suppression, thyroid disease, elevated cholesterol, pregnancy complications, and increased cancer risk particularly kidney and testicular cancers based on studies of highly-exposed populations, lead and other heavy metals that contaminate color cosmetics causing neurotoxicity with no safe exposure threshold and particularly concerning effects on developing brains of fetuses and children, and synthetic fragrances serving as catch-all terms concealing dozens or hundreds of undisclosed chemical compounds including additional phthalates, synthetic musks that bioaccumulate in tissues, allergens causing contact dermatitis, and respiratory irritants triggering asthma attacks.
Beyond these highest-priority concerns, additional problematic ingredients warrant attention including chemical sunscreen filters like oxybenzone and octinoxate demonstrating endocrine disrupting properties and environmental toxicity to coral reefs, coal tar dyes providing color in hair dyes and some cosmetics while being recognized as human carcinogens, triclosan previously common in antibacterial products until FDA restrictions but still appearing in some cosmetics despite hormone-disrupting properties, toluene in nail polish causing neurological effects and reproductive harm through inhalation, hydroquinone in skin-lightening products linked to cancer and organ toxicity, and polyethylene glycols serving as penetration enhancers that may facilitate absorption of other problematic ingredients while potentially being contaminated with ethylene oxide and 1,4-dioxane both recognized as carcinogens. The cumulative exposure from multiple products containing various problematic ingredients creates body burdens exceeding what individual product testing captures, making elimination of highest-priority chemicals across all product categories more important than obsessing over trace amounts in isolated formulations.
Question 2:
Why are toxic ingredients still allowed in cosmetics if they’re dangerous?
Answer 2:
Cosmetic regulations in most countries remain shockingly inadequate with minimal safety testing requirements before products reach consumers, reflecting outdated laws written decades ago when understanding of chemical toxicity and long-term health effects was primitive compared to current scientific knowledge. The FDA in the United States operates under the Federal Food, Drug, and Cosmetic Act passed in 1938 providing remarkably limited authority over cosmetics compared to extensive powers over drugs and medical devices, with no requirements for manufacturers to register products with FDA before marketing, submit safety data demonstrating formulations are safe for intended uses, disclose ingredient lists to regulatory authorities, or report adverse events from consumers experiencing health problems potentially linked to product use. This regulatory framework has banned only eleven ingredients for cosmetic use versus over sixteen hundred substances prohibited in the European Union, revealing massive regulatory gaps allowing chemicals in American products that cannot legally be sold in dozens of other developed nations with stronger consumer protections.
The burden of proof under current American regulations perversely falls on FDA to definitively demonstrate that ingredients cause harm before the agency can restrict use, rather than requiring manufacturers to prove safety before including substances in products people will apply to their bodies. This means chemicals can be widely used for decades while evidence of harm slowly accumulates through scientific research, during which time millions get exposed to substances eventually recognized as dangerous but only after damage is done. Even when concerning evidence emerges, the legal and political process of translating that evidence into regulatory restrictions proves lengthy and contentious as industry groups challenge any restrictions using substantial lobbying resources and legal teams to protect profitable ingredients regardless of health implications. The cosmetic industry’s political influence and campaign contributions ensure regulatory reform faces consistent opposition despite mounting scientific evidence justifying stronger safety standards, creating situations where industry interests consistently outweigh public health concerns in regulatory and political processes determining which chemicals can legally be included in products marketed for daily use.
International regulatory comparisons reveal these inadequacies aren’t inevitable necessities but rather reflect political choices about balancing industry freedom against consumer protection, with numerous other wealthy nations implementing far more robust cosmetic safety requirements including pre-market safety assessments, comprehensive ingredient prohibitions based on health evidence, mandatory adverse event reporting, and stronger enforcement mechanisms. The failure of American regulations to provide comparable protections doesn’t reflect scientific uncertainty since international regulators restricting controversial ingredients cite the same research literature available to American agencies, but rather represents regulatory capture where industry concerns dominate policy decisions despite public health implications.
Question 3:
How can I identify harmful ingredients in my beauty products?
Answer 3:
Reading ingredient lists carefully and learning to recognize common problematic chemicals represents the foundation of identifying harmful ingredients in beauty products you currently use or are considering purchasing. Focus initially on memorizing the highest-priority ingredient categories to avoid including parabens recognizable by names ending in -paraben such as methylparaben, propylparaben, butylparaben, and ethylparaben, phthalates typically including phthalate in names like dibutyl phthalate or diethyl phthalate though they also hide behind generic fragrance or parfum designations without disclosure, formaldehyde-releasing preservatives including quaternium-15, DMDM hydantoin, imidazolidinyl urea, diazolidinyl urea, polyoxymethylene urea, sodium hydroxymethylglycinate, and 2-bromo-2-nitropropane-1,3-diol, synthetic fragrances listed simply as fragrance or parfum that conceal dozens of undisclosed chemicals potentially including additional phthalates and other problematic compounds, and chemical sunscreen ingredients particularly oxybenzone and octinoxate that demonstrate endocrine disrupting properties and environmental toxicity.
Beyond memorization of specific ingredient names, utilizing ingredient-checking apps and online databases dramatically expands ability to evaluate products even when unfamiliar with particular chemicals appearing in formulations. The Environmental Working Group’s Skin Deep database allows searching specific products or ingredients to see safety ratings based on hazard assessments incorporating consideration of cancer risks, developmental and reproductive toxicity, allergies and immunotoxicity, and other health concerns, though critics note EWG ratings sometimes emphasize precautionary concerns even when scientific consensus about actual risks remains uncertain. The Think Dirty app provides similar functionality through smartphone scanning of product barcodes delivering immediate ingredient safety ratings, while other resources including EWG’s Healthy Living app, Good Guide, and various clean beauty company databases offer alternative rating systems emphasizing different safety priorities and assessment methodologies. Cross-referencing products across multiple evaluation resources helps identify where different organizations agree versus where rating disagreements reflect genuine scientific uncertainty or different philosophical approaches to precautionary assessment.
For products containing ingredients that databases flag as concerning, investigating the specific evidence underlying concern enables informed decisions about whether particular chemicals warrant avoidance for your personal priorities and risk tolerance. Research cited in ingredient assessments may include definitive evidence of serious harm justifying absolute avoidance, suggestive but not conclusive evidence that reasonable people might interpret differently, or highly speculative concerns based on limited preliminary research where uncertainty dominates over established knowledge. Understanding these distinctions prevents both excessive anxiety about marginal concerns that may never manifest as actual harm and complacent dismissal of legitimate risks supported by robust evidence. The goal involves becoming an informed consumer capable of making personal risk trade-offs rather than simply following absolutist rules about ingredients to avoid regardless of context or evidence quality.
Question 4:
Are expensive beauty products safer than drugstore brands?
Answer 4:
Price correlates poorly with ingredient safety as luxury and drugstore brands both frequently contain toxic ingredients despite dramatic price differences, with expensive products often using identical problematic preservatives, fragrances, and chemicals as budget options while charging premium prices justified through marketing, packaging, and brand positioning rather than superior ingredient safety or efficacy. Testing by consumer advocacy organizations consistently finds that drugstore products perform comparably to luxury alternatives in blind tests where brand identities are concealed, while ingredient analysis reveals that prestige brands regularly include parabens, phthalates, synthetic fragrances, and other controversial chemicals just as frequently as mass-market formulations costing ten times less. The luxury beauty industry’s premium pricing reflects factors including expensive advertising campaigns featuring celebrity endorsements, elaborate packaging in heavy glass jars and artistic bottles, department store placement and sales associate services, and brand heritage or prestige positioning rather than meaningful differences in formulation safety or ingredient quality that would justify the substantial cost premiums.
Some affordable clean beauty brands including Acure, Alba Botanica, Desert Essence, and others offer safer formulations at prices competitive with conventional drugstore products, demonstrating that ingredient safety doesn’t necessitate luxury pricing when companies prioritize reformulation over expensive marketing and packaging. Conversely, numerous prestige brands charging premium prices include problematic ingredients in formulations while marketing natural or clean brand positioning that creates false impressions of superior safety without actual formulation differences justifying such perceptions. The lack of correlation between price and safety means consumers paying premium prices for luxury products while assuming expense guarantees safety often expose themselves to identical chemical risks as drugstore users while simply paying more for the privilege.
The most reliable approach involves evaluating ingredient lists directly rather than using price as proxy for safety, with product cost properly reflecting factors like ingredient quality that affects efficacy, formulation elegance influencing sensory experience, and packaging reducing environmental impact rather than assuming expensive equals safer. Some genuinely clean formulations cost more than conventional alternatives due to premium ingredient sourcing or smaller production scales reducing economies of scale, but these price differences reflect real formulation costs rather than pure brand positioning. Conversely, some affordable brands achieve excellent safety profiles through prioritizing reformulation over marketing expense, demonstrating that conscious consumers can build safe routines without luxury budgets while those paying premium prices should verify that formulations justify costs through superior safety or efficacy rather than simply brand prestige.
Question 5:
What are PFAS forever chemicals and why are they in cosmetics?
Answer 5:
PFAS—per- and polyfluoroalkyl substances—represent a class of thousands of synthetic fluorinated chemicals called forever chemicals because they contain carbon-fluorine bonds that rank among the strongest known to science, making these compounds extraordinarily resistant to all natural biological and environmental degradation processes. The extreme stability that makes PFAS valuable for manufacturing purposes creates the fundamental problem that once these chemicals enter ecosystems or human bodies, they persist essentially indefinitely without breaking down into harmless degradation products as most organic chemicals eventually do. This persistence combined with widespread production and use across thousands of consumer and industrial applications has created global contamination where PFAS appear in environments from Arctic ice to deep ocean trenches despite never being directly used in those locations, while biomonitoring studies detect PFAS in blood serum of virtually everyone tested in developed nations confirming ubiquitous human exposure through multiple pathways.
Cosmetic manufacturers add PFAS to beauty products primarily to provide water-resistance and oil-resistance in foundations and liquid makeup, improve product texture and spreadability creating silky smooth application characteristics, enhance wear-time and durability allowing cosmetics to remain in place throughout the day without fading or smudging, and create transfer-resistance preventing makeup from rubbing off onto clothing or other surfaces. Testing by environmental health organizations has detected PFAS in roughly half of makeup products analyzed with particularly high detection rates in waterproof mascara, long-wear liquid lipstick, foundations marketed with stay-on claims, and concealers emphasizing durability, though PFAS can appear in any product category where manufacturers desire the unique performance characteristics these forever chemicals provide. The specific PFAS variants used in cosmetics include ingredients like perfluorohexane, perfluorodecalin, perfluoroperhydrophenanthrene, and polytef among numerous others, appearing under technical chemical names that reveal nothing to average consumers about these being the problematic forever chemicals increasingly recognized as serious environmental and health hazards.
The health concerns associated with PFAS exposure include immune system suppression documented through research showing these chemicals reduce antibody responses to vaccinations in children and adults, thyroid disruption causing altered hormone levels affecting metabolism and development, elevated cholesterol increasing cardiovascular disease risk, pregnancy complications including preeclampsia and reduced birth weight, and increased cancer risk particularly for kidney and testicular cancers based on elevated rates observed in communities with documented high PFAS exposure through contaminated drinking water. The biological mechanisms underlying these health effects involve PFAS binding to proteins throughout the body and interfering with normal cellular processes, disrupting hormone signaling and metabolism, and potentially promoting tumor development through effects on cell growth regulation. The half-lives of major PFAS compounds in human blood range from two to eight years depending on specific chemical structure, meaning even single exposures create body burdens persisting for years or decades while ongoing exposures from cosmetics and other sources cause continuous accumulation throughout life since elimination occurs far slower than intake creating progressive increases in tissue concentrations.
Question 6:
Can natural and organic beauty products contain harmful chemicals?
Answer 6:
Natural and organic labels provide no guarantee of ingredient safety or freedom from harmful chemicals as these marketing terms lack strict legal definitions in cosmetics allowing misleading use that creates false impressions of purity regardless of actual formulation characteristics. Products labeled natural may contain synthetic preservatives, toxic fragrances, and harmful additives alongside plant-derived ingredients since no regulatory standard defines how much natural content qualifies products for natural designation or which synthetic ingredients disqualify products from using this term. Even genuinely natural ingredients derived entirely from plant, animal, or mineral sources can cause allergies, skin sensitivities, or other adverse reactions since natural doesn’t equal non-allergenic or universally safe for all individuals. Poison ivy provides an obvious example of natural plant material causing severe reactions despite being completely natural without any synthetic chemical contamination, illustrating how nature produces numerous compounds that harm humans despite their organic botanical origins.
Organic certification in cosmetics carries varying meanings depending on which standard applies, with requirements ranging from merely seventy percent organic content of agricultural ingredients—not counting water and minerals that don’t factor into organic percentage calculations—to ninety-five percent or one hundred percent organic in stricter standards. Products labeled made with organic ingredients typically contain lower organic percentages than those carrying actual organic certification seals, exploiting consumer confusion about certification distinctions to suggest superior purity through featuring organic ingredients prominently in marketing without formulation meeting rigorous organic standards. Crucially, organic certification addresses agricultural practices and pesticide residues rather than overall ingredient safety or toxicity, meaning organic products can contain synthetic preservatives, fragrances, and other chemicals that concern consumers focused on ingredient safety rather than farming methods. A product might be certified organic while still including parabens, phthalates, or synthetic fragrances if those ingredients represent less than five percent of formulation and the remaining ninety-five percent consists of organically-farmed plant ingredients.
The most significant concern involves natural and organic products potentially containing harmful ingredients precisely because manufacturers and consumers incorrectly assume natural equals safe requiring less vigilance about ingredient selection. Some plant-derived ingredients pose legitimate safety concerns including essential oils that can cause photosensitivity making skin vulnerable to sun damage, botanical extracts triggering allergic reactions in sensitive individuals, and natural preservatives like phenoxyethanol that some consumers wish to avoid despite plant origins. Additionally, natural products using minimal or inadequate preservation can develop dangerous bacterial or fungal contamination during storage since natural origin doesn’t protect against microbial growth in water-containing formulations, with several incidents documented where natural products harbored pathogenic bacteria causing serious infections including cases of Pseudomonas contamination causing corneal ulcers from contaminated natural mascara.
The most reliable approach involves reading complete ingredient lists and evaluating specific chemicals present rather than trusting natural or organic marketing claims as sufficient safety verification. Genuinely safe natural products do exist when companies carefully select proven safe plant ingredients, implement adequate preservation preventing contamination, avoid synthetic chemicals of concern, and provide complete transparency about all ingredients used. However, these formulation characteristics require verification through ingredient review rather than assumption based on natural positioning since the term means little without independent confirmation that formulations actually avoid problematic ingredients regardless of whether those ingredients derive from synthetic or natural sources.
Question 7:
What health problems are linked to toxic beauty ingredients?
Answer 7:
Toxic cosmetic chemicals contribute to health problems spanning multiple organ systems and disease categories based on extensive scientific research documenting various pathways through which these substances harm human health. Hormone disruption represents one of the most significant concerns with numerous cosmetic ingredients including parabens, phthalates, chemical sunscreen filters like oxybenzone, and others demonstrating endocrine disrupting properties through mimicking or blocking natural hormones. This interference with normal hormone signaling potentially affects reproductive development causing abnormalities in sexual development and fertility problems particularly when exposure occurs during sensitive periods like fetal development and puberty, influences metabolic regulation contributing to obesity and diabetes through disrupted hormone control of appetite and energy balance, impacts thyroid function affecting metabolism and brain development, and alters breast tissue development possibly contributing to rising breast cancer rates in populations where hormonally-driven cancers have increased dramatically over recent decades.
Cancer risks from cosmetic ingredients are documented for several chemicals including formaldehyde classified as a known human carcinogen by international health authorities based on evidence linking inhalation exposure to nasopharyngeal cancer, coal tar dyes used in hair coloring products and some cosmetics that contain polycyclic aromatic hydrocarbons recognized as carcinogenic, and PFAS forever chemicals associated with elevated kidney and testicular cancer rates in highly-exposed populations. The carcinogenic mechanisms vary across chemicals with some acting as mutagens directly damaging DNA and causing genetic mutations that can initiate cancer development, others functioning as tumor promoters that enhance growth of existing cancer cells without directly initiating tumor formation, and still others disrupting hormone signaling in ways that promote hormone-dependent cancers like breast and reproductive cancers. The long latency periods between chemical exposures and cancer diagnosis—often decades for solid tumors—complicate establishing definitive causal links in human populations despite strong mechanistic evidence from laboratory studies demonstrating how specific chemicals can contribute to cancer development.
Allergic reactions and skin sensitivities develop from cosmetic ingredients particularly fragrances and preservatives that rank among leading causes of allergic contact dermatitis diagnosed by dermatologists. These allergic responses occur when repeated exposure sensitizes immune systems causing subsequent exposures to trigger inflammatory reactions ranging from mild itching and redness to severe blistering and painful rashes requiring medical treatment. The allergen triggering sensitivity in one individual may cause no reaction in others since allergy development depends on personal immune system characteristics and prior exposure history, but certain chemicals including fragrance ingredients and formaldehyde act as particularly potent sensitizers affecting substantial percentages of exposed populations. Once sensitization develops, even trace exposures can trigger reactions and sensitivity typically persists lifelong requiring permanent avoidance of problematic ingredients.
Respiratory effects from inhaled cosmetic chemicals concern pulmonologists particularly regarding fragrances that volatilize into air during use and formaldehyde released from preservatives in personal care products. These airborne chemicals trigger asthma attacks in individuals with existing respiratory conditions, aggravate symptoms in people with chemical sensitivities, and potentially contribute to development of new asthma through repeated respiratory tract irritation and sensitization. The volatile organic compounds present in fragrances and other cosmetic ingredients contribute to indoor air quality degradation, with studies measuring elevated VOC concentrations in spaces where fragranced products are used creating continuous low-level respiratory exposure extending beyond the immediate application period.
Neurological effects particularly from heavy metal contamination in cosmetics concern toxicologists since lead causes cognitive impairment and developmental delays in children even at very low exposure levels, while other metals like arsenic and cadmium demonstrate neurotoxic properties. Prenatal and early childhood exposures prove especially concerning since developing brains show particular vulnerability to chemical insults that can cause permanent cognitive and behavioral effects. Reproductive toxicity from chemicals like phthalates affects both female and male reproductive systems through interfering with hormone production and signaling, potentially contributing to declining fertility rates, increasing rates of reproductive tract abnormalities, and pregnancy complications documented across developed nations over recent decades.
Question 8:
How do I transition to safer beauty products without breaking my budget?
Answer 8:
Transitioning to safer beauty products while managing costs requires strategic approaches balancing safety priorities against budget constraints through careful planning and selective replacement rather than attempting immediate wholesale routine transformation that would strain finances and potentially overwhelm through excessive simultaneous change. The most effective strategy involves replacing products gradually as current items are exhausted rather than discarding everything immediately and purchasing complete new routines, allowing spread of costs across months while providing time to research suitable alternatives and verify that chosen products perform adequately before committing to full-size purchases of entire categories. This measured approach typically costs only slightly more than normal product replacement since you’re purchasing items you would need anyway just selecting safer alternatives rather than conventional options, with the incremental cost differences ranging from minimal for some categories to moderate for others depending on price points of specific conventional versus clean beauty brands chosen.
Prioritizing which products to replace first enables focusing initial investments where they deliver maximum safety improvements per dollar spent, with priority ranking considering several factors including exposure frequency and quantity from daily-use items applied to large skin areas creating more significant exposure than occasional products, contact duration where leave-on formulations create longer exposure than rinse-off items briefly applied then removed, absorption characteristics where products applied to thin skin or near mucous membranes allow greater chemical penetration than applications to thick skin on less sensitive areas, and ingredient severity where formulations containing multiple highest-priority problematic chemicals justify earlier replacement than products with isolated minor concerns. This strategic prioritization might lead to replacing daily-use leave-on products like moisturizers, sunscreens, and foundations before concerning yourself with occasional-use items like masks or treatments, and within each category further prioritizing products containing worst ingredient combinations before addressing those with less problematic formulations.
Researching affordable clean beauty brands before purchasing enables identifying safety-focused companies offering reasonable prices rather than assuming clean beauty automatically means luxury pricing. Numerous brands including Acure, Alba Botanica, Desert Essence, Honest Beauty, and others provide genuinely safer formulations at drugstore price points, demonstrating that ingredient safety doesn’t necessitate premium pricing when companies prioritize reformulation over expensive marketing campaigns and elaborate packaging. Comparison shopping across retailers including drugstores, Target, Whole Foods, and online specialists like Thrive Market or Vitacost reveals substantial price variations for identical products, with some retailers marking clean beauty products with significant premiums while others price them competitively. Utilizing coupons, sales, and loyalty programs further reduces costs particularly when planning purchases around promotions rather than buying items at full price due to immediate need.
Simplifying your overall beauty routine eliminates unnecessary products reducing both costs and exposures, with many people discovering through the transition process that they had accumulated numerous items serving redundant or unnecessary functions that elimination actually improves rather than compromises results. Streamlining to essentials focusing on products delivering genuine functional benefits rather than accumulated impulse purchases or items kept out of habit despite rarely using them reduces the total number of products requiring replacement while forcing evaluation of what actually matters in your routine versus what you can happily eliminate entirely.
Making certain items yourself using simple safe ingredients provides another cost-reduction strategy for products amenable to DIY formulation, with basic items like body scrubs, cleansing oils, and simple masks easily replicable at home using food-grade ingredients at costs far below commercial alternatives. However, DIY beauty requires careful research and appropriate caution particularly regarding preservation issues in water-containing formulations, making it most practical for inherently stable oil-based products and items used immediately rather than stored for extended periods. Starting with one or two simple DIY replacements rather than attempting to make everything prevents overwhelming complexity while allowing evaluation of whether homemade beauty fits your priorities and practical realities versus being an appealing idea that proves unsustainable in actual practice.
Question 9:
Are beauty products regulated differently in other countries?
Answer 9:
Cosmetic regulations vary dramatically across countries with some nations implementing robust consumer protection systems requiring pre-market safety assessments and comprehensive ingredient restrictions while others including the United States maintain minimal oversight that allows manufacturers to market products with virtually no regulatory verification of safety. The European Union establishes among the strictest cosmetic regulations globally through requiring pre-market safety assessments conducted by qualified safety assessors before products can be marketed, maintaining a prohibited and restricted ingredients list exceeding sixteen hundred substances identified as carcinogens, mutagens, reproductive toxicants, or otherwise harmful based on scientific evidence reviewed by expert committees, mandating serious adverse effect reporting enabling post-market surveillance of safety problems that emerge through actual consumer use, and requiring detailed ingredient labeling including disclosure of nanomaterials and specific allergen identification beyond general fragrance designations. These comprehensive protections reflect the precautionary principle where potential risks warrant preventive action rather than waiting for conclusive evidence of harm after widespread exposure has occurred.
Canada’s cosmetic regulations similarly prohibit roughly six hundred substances through the Cosmetic Ingredient Hotlist identifying ingredients banned or restricted for safety reasons, while requiring cosmetic manufacturers to report product information including ingredient lists and adverse reactions to Health Canada enabling regulatory oversight of market activities. Japan’s Pharmaceutical and Medical Devices Act covering cosmetics establishes positive lists of permitted ingredients rather than prohibited lists, meaning substances must be specifically approved for cosmetic use based on safety review rather than being allowed freely until evidence forces restrictions. South Korea’s cosmetics act requires safety assessments before product launches, maintains prohibited and restricted ingredient lists similar to other developed nations, implements robust post-market surveillance including mandatory adverse event reporting, and establishes functional cosmetic categories subject to additional review for products making specific claims about effects.
Australia’s cosmetic regulations align largely with European Union standards through the Australian Industrial Chemicals Introduction Scheme, prohibiting similar ingredients and requiring compliance with comprehensive safety assessment protocols before products can be marketed. New Zealand’s regulations similarly follow Australian lead creating largely harmonized cosmetic safety standards across Oceania. Many Asian nations beyond Japan and South Korea have strengthened cosmetic regulations over recent years, implementing increasingly strict safety requirements and ingredient restrictions as scientific understanding of chemical toxicity has advanced and consumer demand for safety protections has increased.
The stark regulatory contrasts reveal particularly clearly when examining specific ingredients that remain legal in American cosmetics despite prohibitions elsewhere, with formulations perfectly acceptable for sale in United States stores unable to be marketed in European Union, Canada, Japan, South Korea, Australia, and numerous other nations due to containing toxic ingredients those countries recognize as unacceptably dangerous based on scientific evidence. American consumers purchasing imported products sometimes discover that international versions of cosmetics differ in formulation from domestic versions because global manufacturers reformulate for different markets to comply with varying regulations, using safer ingredient alternatives in countries with stricter requirements while continuing to use prohibited substances in markets where regulations permit such ingredients.
The practical implication involves recognizing that American cosmetic regulation provides minimal consumer protection compared to international standards, with ingredient legality in US products offering virtually no assurance of safety since regulatory requirements remain so extraordinarily minimal that almost anything short of acute poisoning passes without restriction. This regulatory inadequacy doesn’t reflect scientific uncertainty about problematic ingredients since international regulators restricting them cite the same research literature available to American authorities, but rather represents political choices about prioritizing industry freedom over consumer protection despite public health implications. Until American regulations adopt standards comparable to international best practices, consumers must educate themselves and make informed protective choices rather than relying on regulatory oversight that fundamentally fails to provide adequate protection.
Question 10:
What ingredients should I absolutely avoid in beauty products?
Answer 10:
The highest-priority ingredients warranting absolute avoidance based on strongest scientific evidence of serious health risks include parabens in all forms recognizable by names ending in -paraben including methylparaben, propylparaben, butylparaben, and ethylparaben that function as endocrine disruptors mimicking estrogen and accumulating in breast tissue with documented correlations between exposure and reproductive effects, phthalates particularly dibutyl phthalate and diethyl phthalate that hide both behind explicit ingredient listings and within undisclosed fragrance formulations while causing reproductive system abnormalities and developmental harm especially when exposure occurs during pregnancy, formaldehyde and all formaldehyde-releasing preservatives including quaternium-15, DMDM hydantoin, imidazolidinyl urea, diazolidinyl urea, polyoxymethylene urea, sodium hydroxymethylglycinate, and 2-bromo-2-nitropropane-1,3-diol since formaldehyde is definitively classified as a human carcinogen causing nasopharyngeal cancer through inhalation exposure, and PFAS forever chemicals identifiable through names including fluoro- prefix or other fluorinated compound indicators that persist indefinitely in human organs and environment while contributing to immune suppression, thyroid disease, cancer risk, and numerous other health problems.
Additional ingredients meriting absolute avoidance include synthetic fragrances listed generically as fragrance or parfum that conceal dozens to hundreds of undisclosed chemicals potentially including additional phthalates, synthetic musks, allergens, and respiratory irritants without any requirement for ingredient disclosure or safety verification, chemical sunscreen ingredients particularly oxybenzone and octinoxate that demonstrate endocrine disrupting properties while also causing environmental damage to coral reefs and aquatic ecosystems, coal tar dyes providing color in hair dyes and some cosmetics while containing polycyclic aromatic hydrocarbons recognized as carcinogenic, triclosan previously ubiquitous in antibacterial soaps and still appearing in some cosmetics despite FDA restrictions and documented hormone-disrupting properties plus contribution to antibiotic resistance, toluene used as solvent in nail polish and other products causing neurological effects and reproductive harm through inhalation exposure, and hydroquinone in skin-lightening products linked to cancer and organ toxicity despite continued use in numerous skin care formulations marketed for treating hyperpigmentation.
Heavy metals present as contaminants rather than intentional ingredients deserve avoidance through choosing products from manufacturers committed to rigorous testing and purity verification, with particular concern about lead contamination in lipsticks and color cosmetics, arsenic in mineral makeup and pigments, cadmium in yellow, orange, and red colorants, and other metallic elements that bioaccumulate causing neurological damage, cancer risks, and organ toxicity through chronic exposure. Since current regulations establish no mandatory testing or maximum contamination limits for heavy metals in cosmetics, avoiding contamination requires either patronizing brands voluntarily testing and disclosing results or selecting products using synthetic colorants rather than mineral-derived pigments that represent primary contamination source.
Polyethylene glycols identified by PEG prefix followed by numbers like PEG-8 or PEG-100 serve various functions including moisturizing and penetration enhancement, potentially facilitating absorption of other ingredients through compromised skin while also being subject to contamination with ethylene oxide and 1,4-dioxane both recognized as probable carcinogens during PEG manufacturing. Sodium lauryl sulfate and sodium laureth sulfate used as surfactants and foaming agents in cleansers cause skin irritation particularly in sensitive individuals while sodium laureth sulfate specifically may be contaminated with 1,4-dioxane requiring ethoxidation removal treatment that some manufacturers skip.
The practical challenge involves recognizing that absolutely avoiding every concerning ingredient proves nearly impossible in modern life given ubiquitous chemical exposures from environment, food, and consumer products beyond cosmetics, requiring acceptance that harm reduction through eliminating highest-priority chemicals provides achievable improvement even if perfect purity remains impossible. Focusing elimination efforts on ingredients with strongest evidence of serious health effects delivers meaningful exposure reduction while avoiding paralysis from attempting to avoid every chemical anyone anywhere has ever suggested might pose any theoretical concern however speculative or poorly supported by evidence.
Articles related:
- Complete Skincare Routine for Beginners 2026: Step-by-Step Guide to Glowing Skin from AliExpress - Budget-Friendly from $8!
- Best Anti-Aging Serums to Reduce Visible Signs of Aging in 2026: Transform Your Skin
- Natural Makeup Look Tutorial: The Complete Guide to Effortless Beauty
Other articles on our website:
- Car Air Purifiers That Work: Science-Backed Testing 2026
- Statement Jewelry Pieces: The Complete Guide to Bold Accessories That Transform Your Style
- Top Laptop Recommendations for 2026: Complete Guide to Best Laptops for Every Budget and Need
- The Ultimate Guide to the Best Fitness Trackers: Transform Your Health Journey with Smart Wearable Technology
- Secret farming: How city dwellers are trying to raise certain animals without anyone knowing
- Air Fryer vs Traditional Oven vs Microwave: Which Actually Saves Money and Time? (6-Month Test)
- How to Build a Capsule Wardrobe: The Complete Guide to Timeless Style and Effortless Fashion
Tags
📧 Get More Articles Like This
Subscribe to receive product reviews and buying guides in your inbox!
We respect your privacy. Unsubscribe at any time.